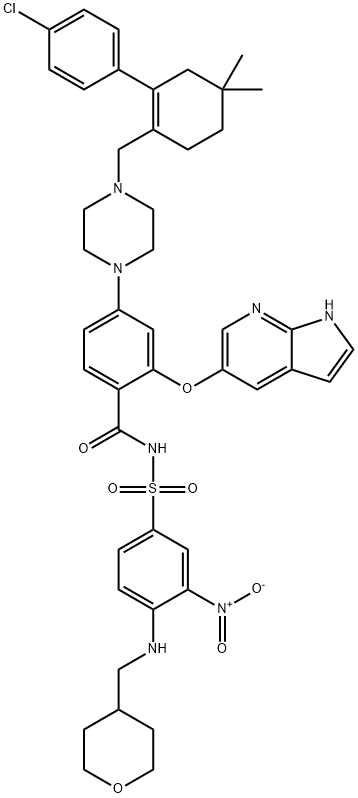
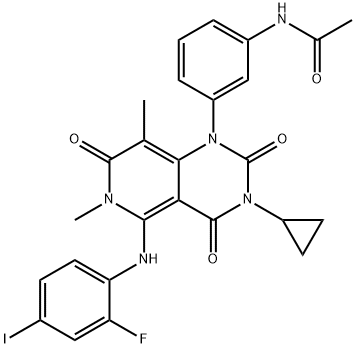
ABT-199 (GDC-0199) , ≥99% , 1257044-40-8
CAS NO.:1257044-40-8
Empirical Formula: C45H50ClN7O7S
Molecular Weight: 868.44
MDL number: MFCD23160052
EINECS: 820-130-9
| Pack Size | Price | Stock | Quantity |
| 5MG | RMB79.20 | In Stock |
|
| 10mg | RMB127.20 | In Stock |
|
| 25MG | RMB239.20 | In Stock |
|
| 50mg | RMB383.20 | In Stock |
|
| 100MG | RMB615.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | >150°C (dec.) |
| Density | 1.340±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly) |
| form | Yellow solid. |
| pka | 4.09±0.10(Predicted) |
| color | Light Yellow to Yellow |
| InChIKey | LQBVNQSMGBZMKD-UHFFFAOYSA-N |
| SMILES | C(NS(C1=CC=C(NCC2CCOCC2)C([N+]([O-])=O)=C1)(=O)=O)(=O)C1=CC=C(N2CCN(CC3CCC(C)(C)CC=3C3=CC=C(Cl)C=C3)CC2)C=C1OC1=CN=C2NC=CC2=C1 |
Description and Uses
Venetoclax, codeveloped by AbbVie (previously Abbott Laboratories) and Genentech/ Roche, was approved in the US for treatment of patients with chronic lymphocytic leukemia (CLL). To meet qualifications for venetoclax treatment, patients must have received prior therapy and possess the 17p deletion genetic mutation, as determined by USFDA testing. Venetoclax functions as a selective inhibitor of B cell lymphoma subtype 2 (BCL-2), which is often overexpressed on malignant cells and thus leads to impairment of the apoptotic pathway. Along these lines, the orally dosed small molecule drug restores the ability of malignant cells to undergo apoptosis as its mechanism of action.90 Although other BCL-2 inhibitors are known, development of similar agents such as navitoclox have been pursued and halted due to undesired inhibition of BCL-XL, leading to significant thrombocytopenia and demonstrating the need for more selective inhibitors. Venetoclax is also currently being considered for approval in Europe and Canada for similar indications and is in various stages of development for the treatment of non-Hodgkin lymphomas (NHL), acute myeloid leukemia (AML), multiple myeloma (MM), and several other disorders, either as a combination therapy or a stand-alone treatment.
ABT 199 (>99%) is a potent and selective BCL-2 inhibitor that achieves potent antitumour activity while sparing platelets. It’s practical application is to treat chronic lymphocytic leukaemic cells and estrogen receptor-positive breast cancer.
Safety
| Symbol(GHS) |  GHS08 |
| Signal word | Danger |
| Hazard statements | H361-H372 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P260-P264-P270-P314-P501 |