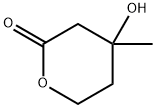
DL-Mevalonolactone , 98% , 674-26-0
Synonym(s):
(±)-β-Hydroxy-β-methyl-δ-valerolactone;(±)-3-Hydroxy-3-methyl δ-valerolactone;DL -Mevalolactone;DL -Mevalonic acid lactone
CAS NO.:674-26-0
Empirical Formula: C6H10O3
Molecular Weight: 130.14
MDL number: MFCD00006648
EINECS: 211-615-0
| Pack Size | Price | Stock | Quantity |
| 10mg | RMB36.80 | In Stock |
|
| 50mg | RMB126.40 | In Stock |
|
| 250mg | RMB423.20 | In Stock |
|
| 1g | RMB1064.00 | In Stock |
|
| 5g | RMB3192.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 28 °C(lit.) |
| Boiling point: | 145-150 °C5 mm Hg(lit.) |
| alpha | -1.0~+1.0°(20℃/D)(c=5, C2H5OH) |
| Density | 1.1066 (rough estimate) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | -20°C |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Ethyl Acetate, Methanol (Slightly) |
| form | Oil |
| pka | 13.57±0.20(Predicted) |
| color | Pale Yellow to Dark Brown |
| Merck | 14,6163 |
| BRN | 80960 |
| Stability: | Hygroscopic |
| InChIKey | JYVXNLLUYHCIIH-UHFFFAOYSA-N |
| CAS DataBase Reference | 674-26-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Dl-mevalonic acid lactone(674-26-0) |
Description and Uses
DL-Mevalonolactone is the δ-lactone form of mevalonic acid, a precursor in the mevalonate pathway. It induces depolarization and swelling, decreases NADPH content and aconitase (ACO) activity, and increases malondialdehyde (MDA) production in calcium-loaded rat brain mitochondria. DL-Mevalonolactone reverses decreases in glucose uptake induced by simvastatin (Item Nos. 10010344 | 10010345) in L6 myotubes. Tissue levels and urinary excretion of DL-mevalonolactone are increased in patients with mevalonic aciduria, a disorder characterized by a deficiency in mevalonic kinase activity.
(±)-Mevalonolactone is used to:
- study the effect of statin on the prenylation of Ras and Rho GTPases
- analyse the isoprenoid biosynthesis pathways in Listeria monocytogenes
- study the the effects of statins on proliferation and migration of HUVECs (HGF-induced human umbilical vein endothelial cells)
Safety
| WGK Germany | 3 |
| F | 3-10 |
| HS Code | 29322090 |