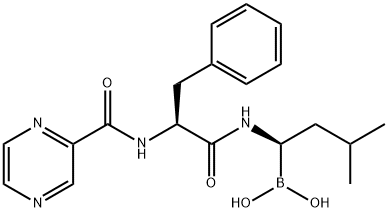
Bortezomib (PS-341) , ≥98% , 179324-69-7
Synonym(s):
;Bortezomib - CAS 179324-69-7 - Calbiochem
CAS NO.:179324-69-7
Empirical Formula: C19H25BN4O4
Molecular Weight: 384.24
MDL number: MFCD09056737
EINECS: 605-854-3
| Pack Size | Price | Stock | Quantity |
| 5MG | RMB31.20 | In Stock |
|
| 25MG | RMB74.40 | In Stock |
|
| 100MG | RMB160.00 | In Stock |
|
| 500mg | RMB557.60 | In Stock |
|
| 1g | RMB888.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 122-124°C |
| Density | 1.214 |
| RTECS | ED7771666 |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in chloroform, dimethyl sulfoxide, ethanol and methanol. |
| form | solid |
| pka | 9.66±0.43(Predicted) |
| color | White |
| Stability: | Hygroscopic and Moisture Sensitive |
| CAS DataBase Reference | 179324-69-7(CAS DataBase Reference) |
Description and Uses
Bortezomib, a potent ubiquitin proteasome (26S) inhibitor (Ki=0.6 nM), was launched in the US as a treatment for multiple myeloma. This proteasome is required for the proteolytic degradation of the majority of cellular proteins and is present in all cells. It is required for the control of inflammatory processes, cell cycle regulation and gene expression and is a novel target in cancer treatment. Bortezomib is a N-acyl-pseudodipeptidyl boronic acid and formulated as a mannitol ester. Aldehyde containing peptides are also proteasome inhibitors, but lack chiral stability (racemization) and selectivity against other proteases including cysteine proteases. Replacement of the aldehyde moiety by a boronic acid avoids these shortcomings and provides some measure of selective proteasome inhibition relative to many other serine proteases. It is prepared by coupling the pinanediol ester of leucine boronic acid with N-tert-Bocphenylalanine utilizing O-(1H-benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate (TBTU) as the coupling agent. The tert-Boc protecting group is then cleaved from the dipeptide and pyrazinecarboxylic acid is coupled to form the terminal amide. Hydrolysis of the boronate ester is accomplished via a two-phase transesterification procedure using isobutyl boronic acid and aqueous extraction. In a study of patients who had received at least two prior therapies and demonstrated disease progression on their most recent therapy, about twenty eight percent showed a response to bortezomib. The response lasted a median time of one year. Another trial in 54 patients with relapsed multiple myeloma showed similar responses. It is dosed intravenously at an exposure of 1.3 mg/m2/dose twice weekly for two weeks followed by a 10-day drug-holiday. At therapeutic doses, the plasma drug levels were reported to drop to near detection limits within minutes of intravenous dosing. Based upon an ex vivo proteasome activity assay using blood cells, the pharmacodynamic half-life ranged from 9 to 15 h in patients with advanced malignancies.
A potent, highly selective and reversible inhibitor of the 20S proteasome, Bortezomib is used as an antineoplastic agent, which controls the growth of cancer cells. It is also useful in the treatment of multiple myeloma.
Safety
| Symbol(GHS) |   GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H300-H310-H330-H372 |
| Precautionary statements | P301+P310a-P304+P340-P320-P330-P405-P501a |
| Risk Statements | 23/24/25-48/23/24/25 |
| Safety Statements | 9-27-36/37-45-60 |
| HazardClass | 6.1 |
| HS Code | 29339900 |
| Hazardous Substances Data | 179324-69-7(Hazardous Substances Data) |