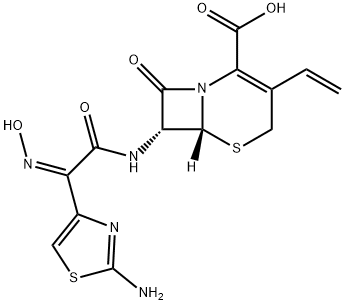
Cefdinir , ≥97%(HPLC) , 91832-40-5
Synonym(s):
[6R-[6α-7beta(Z)]]-7-[[(2-Amino-4-thiazolyl)(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;syn-7-[2-(2-amino-4-thiazolyl)-2-hydroxyiminoacetamido]-3-vinyl-3-cephem-4-carboxylic acid;BMY-28488;FK-482
CAS NO.:91832-40-5
Empirical Formula: C14H13N5O5S2
Molecular Weight: 395.41
MDL number: MFCD00865030
EINECS: 643-088-1
| Pack Size | Price | Stock | Quantity |
| 250mg | RMB191.20 | In Stock |
|
| 1G | RMB495.20 | In Stock |
|
| 5G | RMB1759.20 | In Stock |
|
| 25g | RMB3800.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | >180°C dec. |
| Density | 1.89±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | dilute HCl: slightly soluble |
| pka | 9.70(at 25℃) |
| form | solid |
| color | Pale Yellow to Light Yellow |
| Water Solubility | Soluble in water |
| λmax | 295nm(DMSO)(lit.) |
| Merck | 14,1920 |
| BCS Class | 4 |
| InChIKey | RTXOFQZKPXMALH-GHXIOONMSA-N |
| SMILES | N12[C@@]([H])([C@H](NC(/C(/C3=CSC(N)=N3)=N\O)=O)C1=O)SCC(C=C)=C2C(O)=O |
| CAS DataBase Reference | 91832-40-5(CAS DataBase Reference) |
Description and Uses
Cefdinir is a cephalosporin antibiotic. It is active against numerous Gram-positive and Gram-negative bacteria, including β-lactamase-producing E. coli, K. oxytoca, K. pneumoniae, and P. aeruginosa clinical isolates (MICs = 0.25-16 μg/ml). Cefdinir is protective against sepsis induced by strains of S. aureus or H. influenzae in mice with 50% protective dose (PD50) values of 2.7-35 and 3.1-5.8 mg/kg, respectively. Formulations containing cefdinir have been used in the treatment of Gram-positive and Gram-negative infections.
A broad spectrum antibiotic targeting both Gram-positive and Gram-negative pathogens
Safety
| Symbol(GHS) |  GHS08 |
| Signal word | Danger |
| Hazard statements | H317-H334 |
| Precautionary statements | P261-P272-P280-P284-P302+P352+P333+P313+P363-P304+P340+P342+P311-P501 |
| WGK Germany | 3 |
| RTECS | XI0367250 |
| HS Code | 2941906000 |
| Toxicity | LD50 orl-rat: >5600 mg/kg IYKEDH 23,93,1992 |