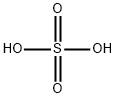
Sulfuricacid , 95%-98% , 7664-93-9
Synonym(s):
aa;Sulfuric acid;Sulfuric acid solution
CAS NO.:7664-93-9
Empirical Formula: H2O4S
Molecular Weight: 98.08
MDL number: MFCD00282010
EINECS: 231-639-5
PRODUCT Properties
| Melting point: | 10°C |
| Boiling point: | ~290 °C (lit.) |
| Density | 1.840 g/mL at 25 °C (lit.) |
| vapor density | <0.3 (25 °C, vs air) |
| vapor pressure | 1 mm Hg ( 146 °C) |
| Flash point: | 11 °C |
| storage temp. | no restrictions. |
| solubility | H2O: soluble |
| form | Viscous Liquid |
| pka | -3-2(at 25℃) |
| color | Pale yellow to slight tan |
| Specific Gravity | 1.84 |
| Odor | Odorless |
| PH | 2.75(1 mM solution);1.87(10 mM solution);1.01(100 mM solution); |
| Water Solubility | miscible |
| Sensitive | Hygroscopic |
| Merck | 14,8974 |
| Exposure limits | TLV-TWA air 1 mg/m3 (ACGIH, MSHA,
and OSHA); TLV-STEL 3 mg/m3 (ACGIH).
. |
| Dielectric constant | 84.0(20℃) |
| Stability: | Stable, but reacts with moisture very exothermically, which may enhance its ability to act as an oxidizing agent. Substances to be avoided include water, most common metals, organic materials, strong reducing agents, combustible materials, bases, oxidising agents. Reacts violently with water - when diluting concentrated acid, carefully and slo |
| LogP | -1 at 25℃ |
| CAS DataBase Reference | 7664-93-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulfuric acid(7664-93-9) |
| EPA Substance Registry System | Sulfuric acid (7664-93-9) |
Description and Uses
Reactivity
Sulfuric acid is very reactive and dissolves most metals, it is
a concentrated acid that oxidizes, dehydrates, or sulfonates
most organic compounds, often causes charring.
Sulfuric acid reacts violently with alcohol and water to release
heat. It reacts with most metals, particularly when diluted with
water, to form flammable hydrogen gas, which may create an
explosion hazard. Sulfuric acid is not combustible, but it is
a strong oxidizer that enhances the combustion of other substances,
does not burn itself. During fire, poisonous gases are
emitted. Hazardous decomposition products are as follows:
sulfur dioxide, sulfur trioxide, and sulfuric acid fumes.
Note: Use great caution in mixing with water due to heat
release that causes explosions. Always add the acid to water,
never the reverse.
Where Found
l Car battery acid
l Certain detergents
l Chemical munitions
l Some fertilizers
l Some toilet bowl cleaners
Derivation
Sulfuric acid is made from sulfur, pyrite (FeS2), hydrogen
sulfide, or sulfur-containing smelter gases by the contact process
(vanadium pentoxide catalyst). The first step is combustion of
elemental sulfur, or roasting of iron pyrites, to yield sulfur
dioxide. Then follows the critical reaction, catalytic oxidation of
sulfur dioxide to sulfur trioxide.
Sulfuric Acid is an acidulant that is a clear, colorless, odorless liquid with great affinity for water. it is prepared by reacting sulfur dioxide with oxygen and mixing the resulting sulfur trioxide with water, or by reacting nitric oxide with sulfur dioxide in water. it is very cor- rosive. it is used as a modifier of food starch and is used in caramel production and in alcoholic beverages.
Safety
| Symbol(GHS) |  GHS05 |
| Signal word | Danger |
| Hazard statements | H290-H314 |
| Precautionary statements | P234-P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | C,T,F,Xi |
| Risk Statements | 36/38-35-39/23/24/25-23/24/25-11 |
| Safety Statements | 26-30-45-36/37-16 |
| OEB | C |
| OEL | TWA: 1 mg/m3 |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 1 |
| RTECS | WS5600000 |
| F | 3 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 28070010 |
| Hazardous Substances Data | 7664-93-9(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 2.14 g/kg (Smyth) |
| IDLA | 15 mg/m3 |