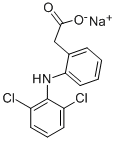
Diclofenac Sodium , ≥99% , 15307-79-6
Synonym(s):
2-[(2,6-Dichlorophenyl)amino]benzeneacetic acid sodium salt;2-[(2,6-Dichlorophenyl)amino]benzeneacetic Acid, Na;Diclofenac Sodium - CAS 15307-79-6 - Calbiochem;Diclofenac sodium salt
CAS NO.:15307-79-6
Empirical Formula: C14H10Cl2NNaO2
Molecular Weight: 318.13
MDL number: MFCD00082251
EINECS: 239-346-4
| Pack Size | Price | Stock | Quantity |
| 5G | RMB31.20 | In Stock |
|
| 25G | RMB52.00 | In Stock |
|
| 100G | RMB128.80 | In Stock |
|
| 500G | RMB369.60 | In Stock |
|
| 2.5kg | RMB1276.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 288-290°C |
| Density | 0.781 g/cm3 |
| storage temp. | room temp |
| solubility | H2O: 50 mg/mL |
| form | White solid |
| pka | 4(at 25℃) |
| color | White to Almost white |
| biological source | synthetic |
| Water Solubility | Soluble in water to 50mg/ml. |
| Merck | 14,3081 |
| BCS Class | 2 |
| Stability: | Stable. |
| InChI | InChI=1S/C14H11Cl2NO2.Na/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19;/h1-7,17H,8H2,(H,18,19);/q;+1/p-1 |
| InChIKey | KPHWPUGNDIVLNH-UHFFFAOYSA-M |
| SMILES | C1(=CC=CC=C1CC([O-])=O)NC1=C(Cl)C=CC=C1Cl.[Na+] |
| CAS DataBase Reference | 15307-79-6(CAS DataBase Reference) |
Description and Uses
Diclofenac is a non-steroidal anti-inflammatory drug (NSAID) and COX inhibitor (IC50s = 0.9-2.7 and 1.5-20 μM, for human COX-1 and COX-2, respectively). It is also an active metabolite of diclofenac methyl ester and diclofenac amide . Diclofenac inhibits release of arachidonic acid (Item Nos. 90010 | 90010.1 | 10006607) induced by A23187 in isolated rat peritoneal neutrophils and macrophages (IC50s = 60 and 10 μM, respectively). Transdermal administration of diclofenac inhibits carrageenan-induced paw edema in rats. Formulations containing diclofenac have been used in the treatment of pain associated with osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis.
Diclofenac sodium is a nonsteroidal anti-inflammatory compound and cyclooxygenase (COX) inhibitor. Oxidation of diclofenac sodium produces the metabolite 4'-hydroxy diclofenac) which demonstrates specific inhibition of Cox-2. Inhibition of Cox by diclofenac and 4'-hydroxy diclofenac suppresses prostaglandin E2 synthesis, producing anti-inflammatory and analgesic effects. Diclofenac is also shown to stabilize the native tetrameric conformation of transthyretin (TTR) fibrils, preventing the formation of insoluble amyloidogenic TTR deposits. Diclofenac Sodium is a substrate of CYP2C9. It is also used as an inhibitor of Cox-1 and Cox-2.
Safety
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H301-H361d-H372-H411 |
| Precautionary statements | P201-P202-P260-P264-P273-P301+P310 |
| Hazard Codes | T,Xi |
| Risk Statements | 25-36/37/38-63 |
| Safety Statements | 22-36/37-45-36-26-60-20 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | AG6330000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29224999 |
| Toxicity | LD50 in mice, rats (mg/kg): ~390, 150 orally (Krupp) |