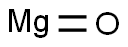Magnesium oxide , AR,98.0% , 1309-48-4
Synonym(s):
Magnesia usta;Magnesii oxidum ponderosum;Magnesium oxide;MG607920
CAS NO.:1309-48-4
Empirical Formula: MgO
Molecular Weight: 40.3
MDL number: MFCD00011109
EINECS: 215-171-9
| Pack Size | Price | Stock | Quantity |
| 100G | RMB24.00 | In Stock |
|
| 250G | RMB39.20 | In Stock |
|
| 500G | RMB55.20 | In Stock |
|
| 2.5KG | RMB140.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 2852 °C (lit.) |
||||||||||||||
| Boiling point: | 3600 °C |
||||||||||||||
| Density | 3.58 |
||||||||||||||
| bulk density | 100kg/m3 |
||||||||||||||
| refractive index | 1.736 |
||||||||||||||
| Flash point: | 3600°C |
||||||||||||||
| storage temp. | no restrictions. |
||||||||||||||
| solubility | 5 M HCl: 0.1 M at 20 °C, clear, colorless |
||||||||||||||
| form | nanopowder |
||||||||||||||
| Specific Gravity | 3.58 |
||||||||||||||
| color | White |
||||||||||||||
| PH | 10.3 (H2O, 20℃)(saturated solution) |
||||||||||||||
| Odor | wh. powd. or cryst., odorless |
||||||||||||||
| Resistivity | 1.3 ∞ 10*15 (ρ/μΩ.cm) |
||||||||||||||
| Water Solubility | 6.2 mg/L (20 ºC), reacts |
||||||||||||||
| semiconductor properties | <111> |
||||||||||||||
| Crystal Structure | Cubic |
||||||||||||||
| λmax | λ: 260 nm Amax: ≤0.040 λ: 280 nm Amax: ≤0.025 |
||||||||||||||
| Sensitive | Air Sensitive |
||||||||||||||
| Merck | 14,5677 |
||||||||||||||
| crystal system | Cube |
||||||||||||||
| Space group | Fm3m |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Dielectric constant | 9.7(0.0℃) |
||||||||||||||
| Exposure limits | ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3 NIOSH: IDLH 750 mg/m3 |
||||||||||||||
| Stability: | Stable. Incompatible with bromine trifluoride, bromine trichloride, phosphorus pentachloride. |
||||||||||||||
| InChIKey | CPLXHLVBOLITMK-UHFFFAOYSA-N |
||||||||||||||
| Hardness, Mohs | 4.0 - 4.5 |
||||||||||||||
| CAS DataBase Reference | 1309-48-4(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | Magnesium monoxide(1309-48-4) |
||||||||||||||
| EPA Substance Registry System | Magnesium oxide (1309-48-4) |
Description and Uses
Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral, often found as a powder, which occurs naturally as periclase and is a source of magnesium . It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2? ions held together by ionic bonding. Magnesium oxide is only very slightly soluble in water but in aqueous media combines quickly with water to form magnesium hydroxide. The majority of magnesium oxide produced today is obtained from the calcination of naturally occurring minerals, magnesite, MgCO3, being the most common. Other important sources of magnesium oxide are seawater, underground deposits of brine and deep salt beds from which magnesium hydroxide [Mg(OH)2] is processed. In medicine, magnesium oxide can be used as an antacid to relieve heartburn, sour stomach, or acid indigestion, as a laxative for short-term, rapid emptying of the bowel (before surgery, for example) and as a mineral supplement used to prevent and treat low amounts of magnesium in the blood. Besides, magnesium oxide also has many nonmedicinal uses. Caustic calcined magnesia is used in a wide range of industrial applications e.g. plastics, rubber, adhesives and acid neutralization. Magnesium oxide with lower chemical activity can be used for fertilizers and animal feed. Dead-burned magnesia and finally fused magnesia can be used for a variety of refractory and electrical applications e.g. furnace lining, crucibles and fireproofing boarding.
manufacture of refractory crucibles, fire bricks, magnesia cements and boiler scale compounds, "powdered" oils, casein glue. Reflector in optical instruments; white color standard. Insulator at low temp.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319 |
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 |
| RTECS | OM3850000 |
| HS Code | 25199099 |
| Hazardous Substances Data | 1309-48-4(Hazardous Substances Data) |
| Toxicity | TCLo inhalation in human: 400mg/m3 |
| IDLA | 750 mg/m3 |