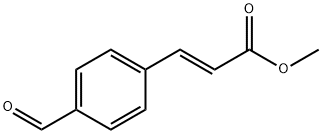
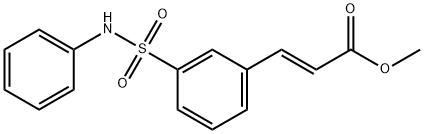
Bilastine , 2mMinDMSO , 202189-78-4
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB159.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 202 °C |
| Boiling point: | 639.1±55.0 °C(Predicted) |
| Density | 1.16±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| Water Solubility | Insoluble in water |
| solubility | DMSO:49.3(Max Conc. mg/mL);106.34(Max Conc. mM) |
| pka | 4.40±0.10(Predicted) |
| form | powder to crystal |
| color | White to Light yellow |
Description and Uses
Bilastine, a potent and selective histamine H1 receptor antagonist, was
approved in Europe in 2010 for the treatment of allergic rhinoconjunctivitis
(AR) and urticaria (hives or skin rash). The original synthesis of bilastine involves alkylation of 2-piperidinyl-1H-benzimidazole with a phenethyltosylate,
the para position of which is substituted with a dimethyloxazoline
moiety serving as a masked carboxylic acid group. Alkylation of the
benzimidazole nitrogen with 2-chloroethyl ethyl ether followed by
unmasking of the oxazoline moiety with sulfuric acid provided bilastine.
In two major clinical trials, bilastine was effective
at relieving allergic rhinitis as assessed by measuring the severity of nasal (obstruction, rhinorrhea, itching, sneezing) and nonnasal (ocular
itching, tearing, ocular redness, itching of ears, and/or palate) symptoms.
Labelled Bilastine. It is a novel, nonsedating H1-antihistamine developed for symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
| HS Code | 2933.39.4100 |

![4-[1-(4,5-DIHYDRO-4,4-DIMETHYL-2-OXAZOLYL)-1-METHYLETHYL]-BENZENEETHANOL](https://img.chemicalbook.com/CAS/GIF/361382-26-5.gif)
![2-(1-(4-(2-(4,4-dimethyl-4,5-dihydrooxazol-2-yl)propan- 2-yl)phenethyl)piperidin-4-yl)-1H-benzo[d]imidazole](https://img.chemicalbook.com/CAS/20180527/GIF/202189-81-9.gif)
![2-Propenoic acid, 3-[3-[(phenylaMino)sulfonyl]phenyl]-, ethyl ester, (2E)-](https://img.chemicalbook.com/CAS/GIF/1137621-29-4.gif)