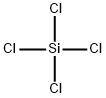
Silicon tetrachloride , 99.9999%metalsbasis , 10026-04-7
Synonym(s):
Silicon tetrachloride;Silicon(IV) chloride, Tetrachlorosilane;STC;Tetrachlorosilane
CAS NO.:10026-04-7
Empirical Formula: Cl4Si
Molecular Weight: 169.9
MDL number: MFCD00011229
EINECS: 233-054-0
PRODUCT Properties
| Melting point: | −70 °C(lit.) |
| Boiling point: | 57.6 °C(lit.) |
| Density | 1.483 g/mL at 25 °C(lit.) |
| vapor density | 5.86 (vs air) |
| vapor pressure | 420 mm Hg ( 37.7 °C) |
| refractive index | 1.413 |
| Flash point: | 57.6°C |
| storage temp. | Store below +30°C. |
| solubility | Miscible with benzene, toluene, chloroform, petroleum ether, carbon tetrachloride, ether and hydrochloric acid. |
| form | Liquid |
| Specific Gravity | 1.483 |
| color | Colorless |
| PH | 1 (5g/l, H2O) |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,8500 |
| Dielectric constant | 2.4(16℃) |
| Stability: | Stability Moisture sensitive - reacts violently with water. Incompatible with water, acids, bases, alcohols, alkali metals, organics, powdered metals. |
| CAS DataBase Reference | 10026-04-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, tetrachloro-(10026-04-7) |
| EPA Substance Registry System | Silane, tetrachloro- (10026-04-7) |
Description and Uses
Chlorosilanes (general formula RnHmSiCl4-n-m, where R is an alkyl, aryl, or olefin group) are compounds in which silicon is bound to between one and four chlorine atoms, bonds with hydrogen and/or organic groups making its total number of bonds up to four. Chlorosilanes react with water, moist air, and steam, producing heat and toxic, corrosive hydrogen chloride fumes. Contact between gaseous hydrogen chloride and metals may release gaseous hydrogen, which is inflammable and explosive. Chlorosilanes react vigorously with oxidizing agents, alcohols, strong acids, strong bases, ketones, and aldehydes.
Chlorosilanes are chemical intermediates used in the production
of silicon and silicon-containing materials, and in the
semiconductor industry; they are also protecting agents for
intermediates in pharmaceutical syntheses. The most important
industrially utilized silicon halides are trichlorosilane and
silicon tetrachloride.
Silicon tetrachloride (SiCl4) can be manufactured by chlorination
of silicon compounds such as ferrosilicon or silicon
carbide, or by heating silicon dioxide and carbon in a stream of
chlorine. It can also be obtained as a by-product in the
production of zirconium tetrachloride, and in the past
substantial quantities were produced by this route, which in
recent decades has lost importance owing to the reduced
demand for zirconium in nuclear facilities. Nowadays, industrial
silicon tetrachloride is produced either by direct reaction of
hydrogen chloride with silicon – this product mainly being
employed as an intermediate in fumed silica production – or as
the by-product of the production of silane for the microelectronics
industry by disproportionation of trichlorosilane.
Safety
| Symbol(GHS) |   GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H301+H331-H314-H335 |
| Precautionary statements | P261-P270-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | C,Xi |
| Risk Statements | 20/21/22-34-40-36/37/38-14-67-37-35-20/22 |
| Safety Statements | 26-7/8-45-36/37/39-28-27 |
| RIDADR | UN 3264 8/PG 2 |
| WGK Germany | 1 |
| RTECS | VW0525000 |
| F | 10-21 |
| TSCA | Yes |
| HS Code | 2812 19 90 |
| HazardClass | 8 |
| PackingGroup | II |
| Hazardous Substances Data | 10026-04-7(Hazardous Substances Data) |