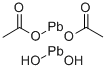
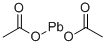Anhydrous lead acetate , 99% , 301-04-2
CAS NO.:301-04-2
Empirical Formula: C4H6O4Pb
Molecular Weight: 325.29
MDL number: MFCD00012452
EINECS: 206-104-4
PRODUCT Properties
| Melting point: | 75 °C (dec.)(lit.) |
| Boiling point: | decomposes at >280℃ [KIR78] |
| Density | 3.3 g/cm3 |
| vapor pressure | 15.7hPa at 25℃ |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Liquid |
| color | Clear colorless |
| Water Solubility | g/100g H2O: 19.7 (0°C), 55.2 (25°C); equilibrium solid phase, Pb(CH3COO)2 ·3H2O [KRU93]; g/100mL H2O: 44.3 (20°C), 221 (50°C) [KIR78] |
| λmax | 260nm(H2O)(lit.) |
| Merck | 14,5397 |
| Solubility Product Constant (Ksp) | pKsp: 2.75 |
| Dielectric constant | 2.5(0.0℃) |
| LogP | -0.17 |
| CAS DataBase Reference | 301-04-2(CAS DataBase Reference) |
| EPA Substance Registry System | Lead(II) acetate (301-04-2) |
Description and Uses
Lead acetate is stable under ordinary conditions of use and storage. Lead acetate is incompatible with bromates, phenol, chloral hydrate, sulphides, hydrogen peroxide, resorcinol, salicylic acid, sulphites, vegetable infusions, alkalis, tannin, phosphates, citrates, chlorides, carbonates, tartrates, and acids. Lead (II) acetate, as well as white lead, has been used in cosmetics throughout history, though this practice has ceased in Western countries. It is still used in men’s hair colouring. Lead (II) acetate paper is used to detect the poisonous gas hydrogen sulphide. The gas reacts with lead (II) acetate on the moistened test paper to form a grey precipitate of lead (II) sulphide.
2 – 1 - Sweetener
Like other lead (II) salts, lead (II) acetate has a sweet taste, which has led to its use as a sugar substitute throughout history. The ancient Romans, who had few sweeteners besides honey, would boil must (grape juice) in lead pots to produce a reduced sugar syrup called defrutum, concentrated again into sapa. This syrup was used to sweeten wine and to sweeten and preserve fruit. It is possible that lead(II) acetate or other lead compounds leaching into the syrup might have caused lead poisoning in anyone consuming it . Lead acetate is no longer used in the production of sweeteners in most of the world because of its recognized toxicity. Modern chemistry can easily detect it, which has all but stopped the illegal use that continued decades after legal use as a sweetener was banned .
2 – 1 - Sweetener2 – 1 – 1 - Resultant deaths
Pope Clement II died in October 1047. A toxicologic examination of his remains conducted in the mid – 20 th century confirmed centuries-old rumors that he had been poisoned with lead sugar.It is not clear if he was assassinated.
In 1787 painter Albert Christoph Dies swallowed, by accident, approximately 21 g of lead acetate. His recovery from this poison was slow and incomplete. He lived with illnesses until his death in 1822 .
Although the use of lead (II) acetate as a sweetener was already illegal at that time, composer Ludwig van Beethoven may have died of lead poisoning caused by wines adulterated with lead acetate.
Mary Seacole applied lead (II) acetate, among other remedies, against an epidemic of cholera in Panama.
Safety
| Symbol(GHS) |   GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H360Df-H373-H410 |
| Precautionary statements | P260-P314-P501-P273-P391-P501 |
| Hazard Codes | T,N |
| Risk Statements | 61-33-48/22-50/53-62 |
| Safety Statements | 53-45-60-61 |
| RIDADR | UN 1616 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | OF8050000 |
| HS Code | 2915.29.5000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Hazardous Substances Data | 301-04-2(Hazardous Substances Data) |
| Toxicity | LD50 i.p. in rats: 15 mg Pb/100g (Bradley, Fredrick) |