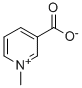
PRODUCT Properties
| Melting point: | 260°C (dec.) |
| Boiling point: | 251.96°C (rough estimate) |
| Density | 1.2528 (rough estimate) |
| refractive index | 1.5030 (estimate) |
| storage temp. | Refrigerator |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | Off-White to Light Beige |
| InChI | InChI=1S/C7H7NO2/c1-8-4-2-3-6(5-8)7(9)10/h2-5H,1H3 |
| InChIKey | WWNNZCOKKKDOPX-UHFFFAOYSA-N |
| SMILES | C1(C([O-])=O)=CC=C[N+](C)=C1 |
| LogP | -3.910 (est) |
Description and Uses
Trigonelline is a bitter alkaloid in coffee which serves to produce important aroma compounds. In terms of concentration trigonelline is higher for arabica than robusta and ranges from about 0.6-1.3% and 0.3-0.9%, respectively.
Trigonelline, the N-methylpyridinium-3-carboxylate, is, after caffeine, the second most important alkaloid of coffee, with about 1% of the green bean. During leaf development, it is synthesized in the leaves and in the fruits' pericarp and accumulated in the seeds. The direct precursors are nicotinic acid and nicotine amide, deriving from the pyridine nucleotide cycle.
Trigonelline is a plant hormone that has diverse regulatory functions with respect to plant cell cycle regulation, nodulation, and oxidative stress, and in helping survival and growth of the plant. It is found in significant quantity in many plants like coffee beans and fenugreek seed. Because it was first isolated from the fenugreek seeds (Trigonella foenum-graecum), the name assigned to it has been “trigonelline.” The chemical formula for trigonelline is C7H7NO2. It is a methylation product of niacin (vitamin B3), and thus is also known as “methylated niacin.” At higher temperatures, trigonelline breaks down to niacin. In addition to trigonelline, fenugreek seeds contain other alkaloids such as gentianine and carpaine.
antihyperglycemic.
Trigonelline is an inhibitor which, like abscicic acid, accumulates in plant cells when the plant becomes dormant or is exposed to stress. This natural inhibitor is chemically related to glycine betaine. It has been found in dormant winter buds of Populus trichocarpa * deltoides trees in the field as well as in salt-stressed micropropagules thereof. Some trigonelline also occurred in non-stressed micropropagules, probably as the result of bioconversion of niacin taken up from the nutrient medium (Bray et al. 1988). Trigonelline preferentially promotes arrest in G2 of the cell cycle and appears to have a growth controlling function during seed germination (Evans and Tramontano 1981).
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Safety Statements | 24/25 |
| HS Code | 29339900 |
| Hazardous Substances Data | 535-83-1(Hazardous Substances Data) |