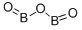Boronoxide , 98% , 1303-86-2
Synonym(s):
BO;Boric anhydride;Boron trioxide;Boric Oxide;Boron Trioxide, Boric oxide
CAS NO.:1303-86-2
Empirical Formula: B2O3
Molecular Weight: 69.62
MDL number: MFCD00011315
EINECS: 215-125-8
| Pack Size | Price | Stock | Quantity |
| 100g | RMB23.20 | In Stock |
|
| 500g | RMB47.20 | In Stock |
|
| 2.5kg | RMB183.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 450 °C(lit.) |
||||||||||||||
| Boiling point: | 1860 °C |
||||||||||||||
| Density | 2.46 g/mL at 25 °C(lit.) |
||||||||||||||
| bulk density | 900-1100kg/m3 |
||||||||||||||
| vapor density | >1 (vs air) |
||||||||||||||
| vapor pressure | 1Pa |
||||||||||||||
| Flash point: | 1860°C |
||||||||||||||
| storage temp. | Inert atmosphere,Room Temperature |
||||||||||||||
| solubility | 36g/l |
||||||||||||||
| form | pellets |
||||||||||||||
| Specific Gravity | 2.46 +/- 0.01 |
||||||||||||||
| color | White |
||||||||||||||
| PH | 4 (10g/l, H2O, 25℃) |
||||||||||||||
| Odor | Odorless |
||||||||||||||
| biological source | human blood |
||||||||||||||
| Water Solubility | 36 g/L (25 ºC) |
||||||||||||||
| Sensitive | Hygroscopic |
||||||||||||||
| Merck | 14,1337 |
||||||||||||||
| crystal system | Three sides |
||||||||||||||
| Space group | P31 |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3 NIOSH: IDLH 2000 mg/m3; TWA 10 mg/m3 |
||||||||||||||
| Stability: | Stable. Moisture sensitive. Incompatible with water. |
||||||||||||||
| InChI | 1S/B2O3/c3-1-5-2-4 |
||||||||||||||
| InChIKey | JKWMSGQKBLHBQQ-UHFFFAOYSA-N |
||||||||||||||
| SMILES | O=BOB=O |
||||||||||||||
| CAS DataBase Reference | 1303-86-2(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | Diboron trioxide(1303-86-2) |
||||||||||||||
| EPA Substance Registry System | Boric oxide (1303-86-2) |
Description and Uses
Boron oxide is a noncombustible, colorless,semitransparent lumps or hard, white, odorless crystals,with slightly bitter taste. Molecular weight=69.64; Boilingpoint=about 1860℃; Freezing/Melting point=about450℃. Hazard Identification (based on NFPA-704 MRating System): Health 2, Flammability 0, Reactivity 0.Moderately soluble in water; solubility=(moderate) 3%;slow reaction.
A saturated solution of H3BO3 contains about 2% of the compound at 0 C, increasing to about 39% at 100 C. The compound also is soluble in alcohol. In preparations, solutions of boric acid are nonirritating and slightly astringent with antiseptic properties. Although no longer used as a preservative for meats, boric acid finds extensive use in mouthwashes, nasal sprays, and eye-hygiene formulations. Boric acid (sometimes with borax) is used as a fire-retardant. A commercial preparation of this type (Minalith) consists of diammonium phosphate, ammonium sulfate, sodium tetraborate, and boric acid. The tanning industry uses boric acid in the deliming of skins where calcium borates, soluble in H2O, are formed. As sold commercially, boric acid is B3O3·3H2O, prepared by adding HCl or H2SO4 to a solution of borax.
Safety
| Symbol(GHS) |  GHS08 |
| Signal word | Danger |
| Hazard statements | H360D |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
| PPE | dust mask type N95 (US), Eyeshields, Gloves |
| Hazard Codes | Xi,T |
| Risk Statements | 36/37/38-61-60 |
| Safety Statements | 26-37/39-45-53 |
| OEB | B |
| OEL | TWA: 10 mg/m3 |
| WGK Germany | 1 |
| RTECS | ED7900000 |
| F | 3-10 |
| TSCA | TSCA listed |
| HS Code | 28100010 |
| Storage Class | 6.1D - Non-combustible acute toxic Cat.3 toxic hazardous materials or hazardous materials causing chronic effects |
| Hazard Classifications | Repr. 1B |
| Hazardous Substances Data | 1303-86-2(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 3150 mg/kg |
| IDLA | 2,000 mg/m3 |