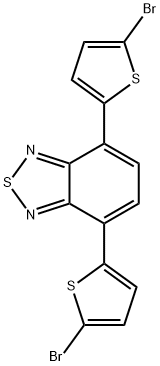
4,7-Bis(5-bromo-2-thienyl)-2,1,3-benzothiadiazole , ≥95.0%(HPLC) , 288071-87-4
CAS NO.:288071-87-4
Empirical Formula: C14H6Br2N2S3
Molecular Weight: 458.21
MDL number: MFCD16619295
| Pack Size | Price | Stock | Quantity |
| 50mg | RMB95.20 | In Stock |
|
| 250MG | RMB319.20 | In Stock |
|
| 1G | RMB871.20 | In Stock |
|
| 5g | RMB3051.20 | In Stock |
|
| 10g | RMB4575.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 245.0 to 249.0 °C |
| Boiling point: | 527.3±45.0 °C(Predicted) |
| Density | 1.898 |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| form | solid |
| pka | -1.63±0.50(Predicted) |
| color | Orange to Brown to Dark purple |
| InChI | InChI=1S/C14H6Br2N2S3/c15-11-5-3-9(19-11)7-1-2-8(10-4-6-12(16)20-10)14-13(7)17-21-18-14/h1-6H |
| InChIKey | ZIIMIGRZSUYQGW-UHFFFAOYSA-N |
| SMILES | N1=C2C(C3SC(Br)=CC=3)=CC=C(C3SC(Br)=CC=3)C2=NS1 |
Description and Uses
4,7-bis(5-bromothiophen-2-yl)benzo[c][1,2,5]thiadiazole is often used as an electron acceptor to synthesize donor–acceptor conjugated polymers. For example, it can be used as a high-purity monomer with 4-(4-((2-ethylhexyl)oxy)phenyl)-4H-dithieno[3,2-b:2′,3′-d]pyrrole (EPDP) through direct (hetero)arylation polymerization and using Pd(OAc)2 as a catalyst system to prepare donor-acceptor (D–A) conjugated polymers. This polymer can be used in the preparation of organic solar cells based on bulk-heterojunction (BHJ) structures[1].
4,7-Bis(2-bromo-5-thienyl)-2,1,3-benzothiadiazole can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and pharmaceutical and chemical production processes.
Safety
| Symbol(GHS) |   GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H301-H318 |
| Precautionary statements | P280-P301+P310+P330-P305+P351+P338+P310 |
| Hazard Codes | T |
| Risk Statements | 25-41 |
| Safety Statements | 26-39-45 |
| RIDADR | UN 2811 6.1 / PGIII |
| WGK Germany | 3 |
| HS Code | 29349990 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Oral Eye Dam. 1 |