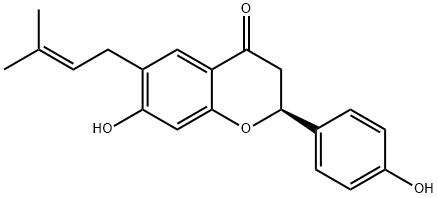
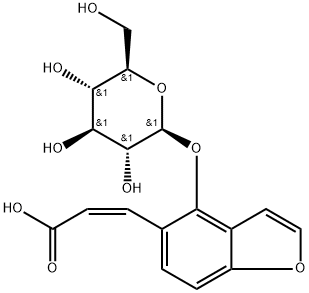
Corylifolin , Analysis of standard products, ≥98.0% , 19879-32-4
Synonym(s):
(2S)-2,3-Dihydro-7-hydroxy-2-(4-hydroxyphenyl)-6-(3-methyl-2-butenyl)-4H-1-benzopyran-4-one;4′,7-Dihydroxy 6-(3-methyl 2-butenyl)flavanone;Corylifolin
| Pack Size | Price | Stock | Quantity |
| 20MG | RMB191.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Boiling point: | 558.3±50.0 °C(Predicted) |
| Density | 1.244 |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | ≤20mg/ml in ethanol;30mg/ml in DMSO;50mg/ml in dimethyl formamide |
| form | crystalline solid |
| pka | 8.27±0.40(Predicted) |
| color | White |
| Major Application | metabolomics vitamins, nutraceuticals, and natural products |
| InChI | InChI=1S/C20H20O4/c1-12(2)3-4-14-9-16-18(23)11-19(24-20(16)10-17(14)22)13-5-7-15(21)8-6-13/h3,5-10,19,21-22H,4,11H2,1-2H3/t19-/m0/s1 |
| InChIKey | OAUREGNZECGNQS-IBGZPJMESA-N |
| SMILES | [C@H]1(C2=CC=C(O)C=C2)OC2=CC(O)=C(C/C=C(/C)\C)C=C2C(=O)C1 |
| CAS DataBase Reference | 19879-32-4 |
Description and Uses
Bavachin is a flavonoid first isolated from seeds of P. corylifolia. It is a phytoestrogen that activates the estrogen receptors ERα and ERβ (EC50s = 320 and 680 nM, respectively). Through this action, bavachin stimulates osteoblast proliferation and differentiation and prevents bone loss following ovariectomy in rats. Bavachin less potently inhibits acyl-coenzyme A:cholesterol acyltransferase (IC50 = 86 μM).
Bavachin has therapeutic potential for type 2 diabetes by activating insulin signaling pathways. Also, it has anabolic and potent anticatabolic biological effects on chondrocytes. Strong inhibitor of human UDP-glucuronosyltransferase (UGT1A1)
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302+H312+H332 |
| Precautionary statements | P261-P264-P280-P301+P312-P302+P352+P312-P304+P340+P312 |
| WGK Germany | WGK 3 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Acute Tox. 4 Dermal Acute Tox. 4 Inhalation Acute Tox. 4 Oral |