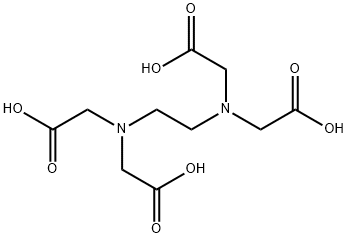
Ethylenediaminetetraacetic acid , Super pure waterless grade, ≥99.5%(titration) , 60-00-4
Synonym(s):
EDTA;Ethylenediaminetetraacetic acid;Ethylenedinitrilotetraacetic acid;EDTA solution;(Ethylenedinitrilo)tetraacetic acid
CAS NO.:60-00-4
Empirical Formula: C10H16N2O8
Molecular Weight: 292.24
MDL number: MFCD00003541
EINECS: 200-449-4
| Pack Size | Price | Stock | Quantity |
| 100G | RMB159.20 | In Stock |
|
| 500G | RMB527.20 | In Stock |
|
| 1KG | RMB903.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 250 °C (dec.) (lit.) |
| Boiling point: | 434.18°C (rough estimate) |
| Density | 1.46 g/cm3 at 20 °C |
| bulk density | 600kg/m3 |
| vapor pressure | <0.013 hPa (20 °C) |
| refractive index | n |
| Flash point: | >400°C DIN 51758 |
| storage temp. | 2-8°C |
| solubility | 3 M NaOH: 100 mg/mL |
| pka | pKa 2 (Uncertain);10.26 (Uncertain) |
| form | crystalline |
| color | White to almost white |
| Odor | Odorless |
| PH Range | 2.5 at 10 g/l at 23 °C |
| PH | 2.5 (10g/l, H2O, 23℃)(slurry) |
| Water Solubility | 0.5 g/L (25 ºC) |
| λmax | λ: 280 nm Amax: ≤0.25 |
| Decomposition | 240 °C |
| Merck | 14,3517 |
| BRN | 1716295 |
| Stability: | Stable. Incompatible with copper, copper alloys, nickel, aluminium, strong oxidizing agents, strong bases |
| Cosmetics Ingredients Functions | CHELATING |
| Cosmetic Ingredient Review (CIR) | Ethylenediaminetetraacetic acid (60-00-4) |
| InChI | 1S/C10H16N2O8/c13-7(14)3-11(4-8(15)16)1-2-12(5-9(17)18)6-10(19)20/h1-6H2,(H,13,14)(H,15,16)(H,17,18)(H,19,20) |
| InChIKey | KCXVZYZYPLLWCC-UHFFFAOYSA-N |
| SMILES | OC(=O)CN(CCN(CC(O)=O)CC(O)=O)CC(O)=O |
| LogP | -0.836 (est) |
| CAS DataBase Reference | 60-00-4(CAS DataBase Reference) |
| NIST Chemistry Reference | N,N'-1,2-Ethane diylbis-(N-(carboxymethyl)glycine)(60-00-4) |
| EPA Substance Registry System | Ethylenediaminetetraacetic acid (60-00-4) |
Description and Uses
Ethylenediaminetetraacetic Acid (EDTA) is a common polydentate ligand. In EDTA, the hydrogen atoms are easily removed
in solution to produce anionic EDTA4-. In its anionic form Ethylenediaminetetraacetic Acid (EDTA) has six binding atoms, two
nitrogen and four oxygen.
Ethylenediaminetetraacetic Acid (EDTA) binds to a metal ion at the six binding sites, wrapping itself around the metal ion,
forming a very stable complex.the strong grasp of Ethylenediaminetetraacetic Acid (EDTA) on the metal ion is analogous
to a crab or lobster clamping down on an object with its claw, hence the name chelation.
Ethylenediaminetetraacetic Acid (EDTA) is such an effective chelating agent because it can deactivate a metal at up to six sites.
EDTA is helps boost a formulation’s preservative system and is also a chelating agent.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313 |
| Hazard Codes | Xi |
| Risk Statements | 36-52/53-36/37/38-36/38 |
| Safety Statements | 26-61-37/39-36 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 2 |
| RTECS | AH4025000 |
| F | 3 |
| Autoignition Temperature | >200 °C |
| TSCA | TSCA listed |
| HS Code | 2922 49 85 |
| HazardClass | 9 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Eye Irrit. 2 |
| Hazardous Substances Data | 60-00-4(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 2580 mg/kg |
| Limited Quantities | 5.0 L (1.3 gallons) (liquid) or 5.0 kg (11 lbs) (solid) |
| Excepted Quantities | Max Inner Pack (30g or 30ml) and Max Outer Pack (1Kg or 1L) |