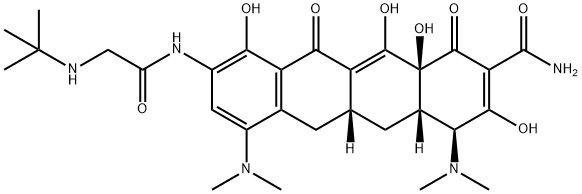
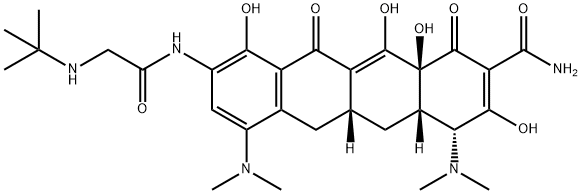
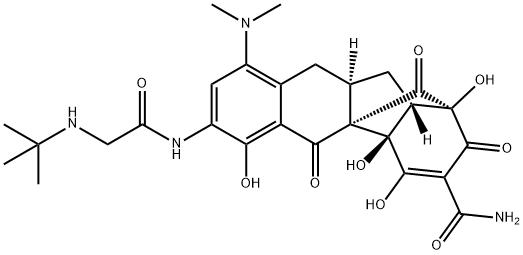
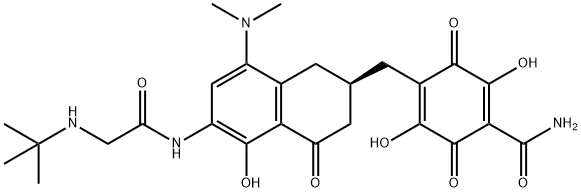
Tigecycline , ≥96% , 220620-09-7
Synonym(s):
9- t-Butylglycylamido-minocycline;9-t-Butylglycylamido-minocycline;9-tert-Butylglycylamidominocycline;Tigecycline;Tigecycline - CAS 220620-09-7 - Calbiochem
CAS NO.:220620-09-7
Empirical Formula: C29H39N5O8
Molecular Weight: 585.65
MDL number: MFCD00935753
EINECS: 685-736-6
| Pack Size | Price | Stock | Quantity |
| 10MG | RMB47.20 | In Stock |
|
| 50MG | RMB87.20 | In Stock |
|
| 250MG | RMB173.60 | In Stock |
|
| 1g | RMB435.20 | In Stock |
|
| 5g | RMB1759.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 164-166°C |
| Boiling point: | 890.9±65.0 °C(Predicted) |
| Density | 1.45±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (up to at least 25 mg/ml). |
| pka | 4.50±1.00(Predicted) |
| form | Orange powder |
| color | Orange |
| Merck | 14,9432 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChIKey | FPZLLRFZJZRHSY-HJYUBDRYSA-N |
| SMILES | C1(=O)[C@]2(O)[C@@]([H])(C[C@@]3([H])C(=C2O)C(=O)C2=C(C(N(C)C)=CC(NC(CNC(C)(C)C)=O)=C2O)C3)[C@H](N(C)C)C(O)=C1C(N)=O |
| CAS DataBase Reference | 220620-09-7(CAS DataBase Reference) |
Description and Uses
The emergence of drug-resistant bacteria has diminished the clinical utility of the
tetracyclines. Research to circumvent the efflux and ribosomal protection mechanisms
of bacteria has led to the development of the glycylcyclines. Tigecycline is
the first glycylcycline antibiotic to launch for the parenteral treatment of
baterial infection, including complicated intra-abdominal and skin infections. Its mechanism of action involves inhibiting protein translation in bacteria by binding
to the 30S ribosomal subunit and blocking entry of amino-acyl tRNA molecules
into the A site of the ribosome to effectively prevent incorporation of amino acid
residues into elongating peptide chains. Presumably, ribosomal protection proteins
are ineffective against tigecycline due to its higher affinity for ribosomal binding
compared to tetracyclines (approximately 16-fold). In addition, tigecycline may be
resistant to efflux mechanisms by either their inability to translocate it across the
cytoplasmic membrane due to steric complications or simply by their failure to
recognize the molecule.
A broad spectrum glycylcycline antibiotic
Safety
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H319-H360D-H372-H410 |
| Precautionary statements | P202-P260-P264-P273-P305+P351+P338-P308+P313 |
| Safety Statements | 24/25 |
| RIDADR | 3077 |
| WGK Germany | WGK 3 |
| RTECS | QI7619500 |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 29419090 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Aquatic Acute 1 Aquatic Chronic 2 Eye Dam. 1 Repr. 1B Skin Sens. 1 |
| Hazardous Substances Data | 220620-09-7(Hazardous Substances Data) |