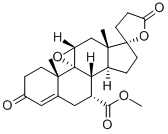
Eplerenone , ≥98% , 107724-20-9
Synonym(s):
Eplerenone;Inspra;Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, gamma-lactone, methyl ester, (7alpha,11alpha,17alpha)-;Eplerenone [USAN];Epoxymexrenone
CAS NO.:107724-20-9
Empirical Formula: C24H30O6
Molecular Weight: 414.49
MDL number: MFCD07783647
EINECS: 600-850-8
| Pack Size | Price | Stock | Quantity |
| 10MG | RMB39.20 | In Stock |
|
| 50MG | RMB70.40 | In Stock |
|
| 250MG | RMB204.00 | In Stock |
|
| 1g | RMB332.80 | In Stock |
|
| 200mg | RMB952.00 | In Stock |
|
| 5g | RMB1015.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 241-243°C |
| alpha | D +5° (c = 0.437 in chloroform) |
| Boiling point: | 597.9±50.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | Store at RT |
| solubility | DMSO: soluble2mg/mL, clear (warmed) |
| form | powder |
| color | white to beige |
| λmax | 240nm(lit.) |
| Merck | 14,3625 |
| Major Application | pharmaceutical |
| InChIKey | UZZAHAKTLYRDOK-MGIDJNSKSA-N |
| SMILES | [C@@]123[C@@]4(C)CCC(=O)C=C4C[C@@H](C(OC)=O)[C@@]1([H])[C@]1([H])CC[C@]4(CCC(=O)O4)[C@]1(C[C@@]2([H])O3)C |&1:0,1,10,15,17,21,27,29,r| |
Description and Uses
Eplerenone derives its antihypertensive effect by blocking the binding of aldosterone at the mineralocorticoid receptor (MR). The drug, which was previously approved only for the oral treatment of hypertension, is now indicated to improve survival of stable patients with left ventricular systolic dysfunction (ejection fraction <40%) and clinical evidence congestive heart failure (CHF) after an acute myocardial infarction. Aldosterone is a key hormone in the renin-angiotensin-aldosterone system (RAAS), which is of critical importance in the development and progression of hypertension, cardiac remodeling and other cardiovascular diseases. The purpose of RAAS is to control sodium, potassium, and fluid volume balance. Aldosterone binds to MRs in both epithelial (e.g. kidney) and nonepithelial (e.g. heart, blood vessels, and brain) tissues and increases blood pressure through induction of sodium reabsorption and possibly other mechanisms. The actions of aldosterone can be blocked by spironolactone (Aldactone ?), a relatively nonselective MR antagonist that has been used in clinical practice for many years. Eplerenone, a structural analog of spironolactone, is a highly selective MR antagonist, with significantly lower affinity for other nuclear receptors. It can be prepared by several related ways, with the key step being the introduction of 11-a-hydroxy group on the steroid scaffold via microbiological conversion. The presence of the 11-a-hydroxy group permits the derivation of the epoxy functionality found in eplerenone. Following oral administration, eplerenone is well absorbed and reaches peak plasma concentrations in~2 h. The bioavailability of eplerenone is 98% and it is cleared predominantly by CYP3A4 metabolism, with an elimination half-life of 4–6 h. Steady state is reached within two days. Eplerenone therapy is typically initiated with 25 mg once daily oral dosing and, if tolerated by the patient, titrated to 50 mg once daily. In a clinical study, eplerenone significantly reduced deaths in congestive heart failure patients after a heart attack, above and beyond standard therapy, including ACE inhibitors and β-blockers. The trial in more than 6600 hospitalized patients demonstrated a 15% reduction in the risk of death for eplerenone compared with placebo, in addition to standard treatment. The most commonly reported adverse events associated with eplerenone are hyperkalemia and increased creatine.
Eplerenone is an aldosterone antagonist with an IC50 of 0.36 μM. It is used as an adjunct in the management of chronic heart failure. It is similar to the diuretic spironolactone, though it may be more specific for the mineralocorticoid receptor and is sp
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| RIDADR | 3077 |
| WGK Germany | 3 |
| HS Code | 29322090 |
| Storage Class | 11 - Combustible Solids |
| Hazardous Substances Data | 107724-20-9(Hazardous Substances Data) |