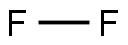A4388212
Fluoride solution , 0.80mg/L,inH2O , 7782-41-4
Synonym(s):
Coagulation factor II;F2;THRB
CAS NO.:7782-41-4
Empirical Formula: F2
Molecular Weight: 38
MDL number: MFCD00050975
EINECS: 231-954-8
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | -220°C |
| Boiling point: | -188°C |
| Density | 1.695(15℃) |
| vapor pressure | >760 mmHg at 20 °C |
| refractive index | 1.000195 |
| storage temp. | -20°C |
| solubility | reacts with H2O |
| form | pale yellow gas |
| color | pale |
| Odor | Strong ozone-like odor detectable at 0.1 to 0.2 ppm |
| biological source | rabbit |
| Water Solubility | reacts |
| Exposure limits | TLV-TWA 1 ppm (~2 mg/m3) (ACGIH and
MSHA), 0.1 ppm (OSHA); IDLH 25 ppm
(NIOSH). |
| Dielectric constant | 1.5(-201℃) |
| Stability: | Stable. Extremely strong oxidant which may react violently with combustible materials, including plastics, reducing agents and organic material. Reacts with water to form corrosive acids. |
| CAS DataBase Reference | 7782-41-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Fluorine(7782-41-4) |
| EPA Substance Registry System | Fluorine (7782-41-4) |
Description and Uses
Fluorine is a highly toxic, pale yellow gas about 1.3 times as heavy as air at atmospheric temperature and pressure. When cooled below its boiling point (-306.8°F or -188.2°C), it is a liquid about 1.5 times as dense as water.
In manufacture of UF6 for nuclear power generation, of SF6 for dielectrics, of fluorinating and metal fluoride compounds.
Safety
| Symbol(GHS) |     GHS04,GHS03,GHS06,GHS05 |
| Signal word | Danger |
| Hazard statements | H270-H314-H330 |
| Precautionary statements | P220-P244-P370+P376-P403-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501 |
| Hazard Codes | T+,C |
| Risk Statements | 7-26-35 |
| Safety Statements | 9-26-36/37/39-45 |
| RIDADR | UN 1045/9192 |
| OEB | C |
| OEL | TWA: 0.1 ppm (0.2 mg/m3) |
| WGK Germany | WGK 1 |
| TSCA | TSCA listed |
| DOT Classification | 2.3, Hazard Zone A (Gas poisonous by inhalation) |
| HazardClass | 2.3 |
| Storage Class | 10 - Combustible liquids |
| Hazardous Substances Data | 7782-41-4(Hazardous Substances Data) |
| Toxicity | LC50 (1 hr) inhalation by rats, mice, guinea pigs: 185, 150, 170 ppm (by vol) (Keplinger, Suissa) |
| IDLA | 25 ppm |