A4607212
Gambogic acid , ≥97%(HPLC) , 2752-65-0
Synonym(s):
Beta-Guttiferrin;Guttatic acid;Guttic acid;Guttic Acid, β-Guttiferin;Beta-Guttilactone
| Pack Size | Price | Stock | Quantity |
| 5MG | RMB163.20 | In Stock |
|
| 25MG | RMB695.20 | In Stock |
|
| 100MG | RMB1991.20 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 88.5°C |
| Boiling point: | 586.06°C (rough estimate) |
| alpha | D20 -685° (methanol) |
| Density | 1.1057 (rough estimate) |
| refractive index | 1.5290 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: ≥10mg/mL |
| form | powder |
| pka | 4.58±0.36(Predicted) |
| color | yellow to orange |
| Stability: | Stable for 1 year as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| InChIKey | GEZHEQNLKAOMCA-XKZIYDEJSA-N |
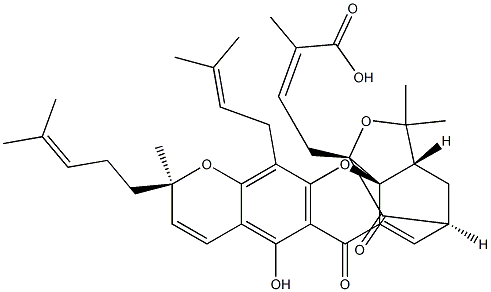
| SMILES | C(O)(=O)/C(/C)=C\C[C@]12C(=O)[C@]3([H])C=C4C(=O)C5=C(O[C@@]41[C@]([H])(C(C)(C)O2)C3)C(C/C=C(\C)/C)=C1O[C@](C)(CC/C=C(\C)/C)C=CC1=C5O |&1:7,10,19,20,35,r| |
| LogP | 6.490 (est) |
Description and Uses
Gambogic acid (2752-65-0) induces apoptosis in a variety of cell lines acting as an antagonist of Bcl-2 family proteins (IC50 < 1 mM)1, however additional targets may contribute to its cytotoxicity. It suppresses cancer invasion and migration by inhibiting TGFb1-induced epithelial-to-mesenchymal transition.2 Potentiates the chemosensitivity of cancer cells to other agents.3 Down-regulates surviving, reversing docetaxel resistance in cancer cells.4
antiinflammatory, cytotoxic, inhibits HeLa cells in vitro;
Safety
| Symbol(GHS) |  GHS06 |
| Signal word | Danger |
| Hazard statements | H301-H315-H319-H335 |
| Precautionary statements | P261-P264-P270-P301+P310-P302+P352-P305+P351+P338 |
| target organs | Respiratory system |
| PPE | Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges |
| Hazard Codes | T |
| Risk Statements | 25-36/37/38 |
| Safety Statements | 26-45 |
| RIDADR | UN 2811 6.1 / PGIII |
| WGK Germany | 1 |
| RTECS | PB9268200 |
| HS Code | 29189900 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Oral Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |
| Toxicity | LD50 unreported in mammal (species unspecified): 55mg/kg |