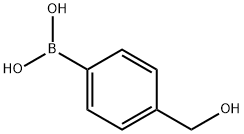
A4708812
4-(Hydroxymethyl)phenylboronic acid(contains varying amounts of Anhydride) , 97% , 59016-93-2
Synonym(s):
α-Hydroxy-p-tolueneboronic acid;4-Hydroxymethylbenzeneboronic acid
| Pack Size | Price | Stock | Quantity |
| 1G | RMB23.20 | In Stock |
|
| 5G | RMB63.20 | In Stock |
|
| 25G | RMB263.20 | In Stock |
|
| 100G | RMB959.20 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 251-256 °C (lit.) |
| Boiling point: | 362.0±44.0 °C(Predicted) |
| Density | 1.25±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| pka | 8.53±0.10(Predicted) |
| form | Crystalline Powder |
| color | White to slightly yellow |
| PH | 4.5 (10g/l, H2O) |
| Water Solubility | 25 g/L |
| BRN | 2831413 |
| InChI | InChI=1S/C7H9BO3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4,9-11H,5H2 |
| InChIKey | PZRPBPMLSSNFOM-UHFFFAOYSA-N |
| SMILES | B(C1=CC=C(CO)C=C1)(O)O |
| CAS DataBase Reference | 59016-93-2(CAS DataBase Reference) |
Description and Uses
Reactant involved in the synthesis of biologically active compounds including:
- Imidazo[4,5-b]pyrazin-2-ones for use as mTOR kinase inhibitors
- Human immunodificiency virus protease inhibitors with activity against resistant viruses
Reactant involved in:
- Suzuki coupling reactions
- Copper-catalyzed transformation from arylboronic acids in water
Involved in the synthesis of polyurethane containing spindle-type chromophores
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313-P261-P280a-P304+P340-P305+P351+P338-P405-P501a |
| PPE | Eyeshields, Gloves, type N95 (US) |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 37/39-26-36 |
| WGK Germany | 3 |
| HazardClass | IRRITANT |
| HS Code | 29319090 |
| Storage Class | 11 - Combustible Solids |