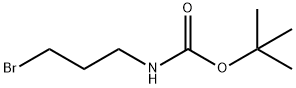
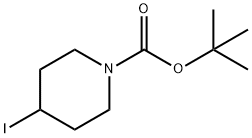
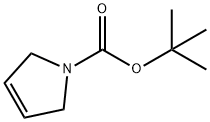
A5061412
3-(<i>tert</i>-Butoxycarbonylamino)propyl Bromide , ≥96.0% , 83948-53-2
Synonym(s):
tert-Butyl N-(3-bromopropyl)carbamate
| Pack Size | Price | Stock | Quantity |
| 250mg | RMB25.60 | In Stock |
|
| 1G | RMB52.80 | In Stock |
|
| 5G | RMB102.40 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 37-39 °C |
| Boiling point: | 285.3±23.0 °C(Predicted) |
| Density | 1.279±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Sparingly), Methanol (Slightly) |
| pka | 12.48±0.46(Predicted) |
| form | Low Melting Solid |
| color | White |
| BRN | 4176344 |
| InChI | InChI=1S/C8H16BrNO2/c1-8(2,3)12-7(11)10-6-4-5-9/h4-6H2,1-3H3,(H,10,11) |
| InChIKey | IOKGWQZQCNXXLD-UHFFFAOYSA-N |
| SMILES | C(OC(C)(C)C)(=O)NCCCBr |
| CAS DataBase Reference | 83948-53-2 |
Description and Uses
3-(Boc-amino)propyl bromide can be used as an alkylating reagent for the synthesis of:
- Benzydamine analogs to be used as activators for soluble guanylate cyclase.
- N-substituted chromenotriazolopyrimidine, human murine double minute 2 (MDM2) inhibitor.
- Protected amines from piperidine derivatives to be further used for synthesis of sulfonamide series.
It can also be used for the post-polymerization quaternization of polymers to synthesize functional cationic polymers and antimicrobial agents.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P301+P312+P330-P302+P352-P305+P351+P338 |
| target organs | Respiratory system |
| PPE | dust mask type N95 (US), Eyeshields, Gloves |
| Hazard Codes | Xn |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-24/25 |
| WGK Germany | 3 |
| F | 10-21 |
| HS Code | 29241990 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Acute Tox. 4 Oral Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |