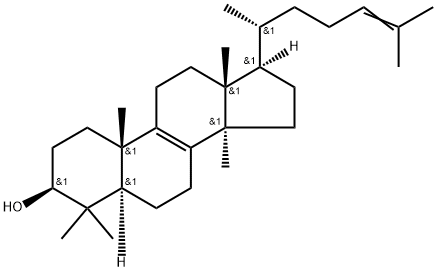
A5296512
lanosta-8,24-dien-3-ol , >95% , 79-63-0
Synonym(s):
3β-Hydroxy-8,24-lanostadiene;8,24-Lanostadien-3β-ol;lanosterin; lanosta-8,24-dienol
CAS NO.:79-63-0
Empirical Formula: C30H50O
Molecular Weight: 426.73
MDL number: MFCD00021108
EINECS: 201-214-9
| Pack Size | Price | Stock | Quantity |
| 1MG | RMB319.20 | In Stock |
|
| 5MG | RMB1039.20 | In Stock |
|
| 25MG | RMB4159.20 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 137 °C |
| alpha | D20 +62.0° (chloroform) |
| Boiling point: | 482.1°C (rough estimate) |
| Density | 0.9600 (rough estimate) |
| refractive index | 1.4910 (estimate) |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| pka | 15.16±0.70(Predicted) |
| form | powder |
| color | white |
| Odor | at 100.00?%. bland |
| Merck | 5360 |
| Cosmetics Ingredients Functions | HAIR CONDITIONING SKIN CONDITIONING - EMOLLIENT ANTISTATIC SKIN CONDITIONING |
| InChIKey | CAHGCLMLTWQZNJ-BQNIITSRSA-N |
| SMILES | [H][C@@]1(CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]4(C)CC[C@H](O)C(C)(C)[C@]4([H])CC3)[C@H](C)CC\C=C(/C)C |
| LogP | 10.521 (est) |
| CAS DataBase Reference | 79-63-0(CAS DataBase Reference) |
Description and Uses
Lanosterol is a naturally-occurring sterol and biosynthetic precursor of several animal, fungal, and protozoan steroids, including cholesterol and ergosterol. Defects in the processing of lanosterol contribute to a wide variety of disorders, including the formation of cataracts. Similarly, certain fungicides act by blocking lanosterol processing by fungi.
Lanosterol has been used:
- as a standard in HPLC for the quantification in testis samples
- in S-adenosyl-L-methionine:Δ24-sterol-C-methyltransferase (SMT) assay
- to treat wild-type cells growing in rich medium to know its effects on Sre1 protein
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P301+P312-P302+P352-P304+P340-P305+P351+P338 |
| PPE | Eyeshields, Gloves, type N95 (US) |
| WGK Germany | 1 |
| RTECS | OE3360000 |
| Storage Class | 11 - Combustible Solids |