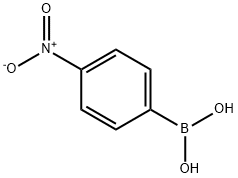
A6245512
4-Nitrophenylboronic Acid (contains varying amounts of Anhydride) , ≥95.0% , 24067-17-2
Synonym(s):
p-nitro-benzeneboronic acid;p-Nitrophenylboronic acid;4-Nitrobenzeneboronic acid
CAS NO.:24067-17-2
Empirical Formula: C6H6BNO4
Molecular Weight: 166.93
MDL number: MFCD00161360
EINECS: 627-647-7
| Pack Size | Price | Stock | Quantity |
| 1G | RMB39.20 | In Stock |
|
| 5G | RMB183.20 | In Stock |
|
| 25G | RMB764.00 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 285-290°C (dec.) |
| Boiling point: | 373.7±44.0 °C(Predicted) |
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMF: 15 mg/ml; DMSO: 15 mg/ml; Ethanol: 15 mg/ml; Ethanol:PBS (pH 7.2) (1:5): 0.16 mg/ml |
| form | Crystalline Powder |
| pka | 7.04±0.10(Predicted) |
| color | White to yellow |
| Water Solubility | Slightly soluble in water. |
| InChI | InChI=1S/C6H6BNO4/c9-7(10)5-1-3-6(4-2-5)8(11)12/h1-4,9-10H |
| InChIKey | NSFJAFZHYOAMHL-UHFFFAOYSA-N |
| SMILES | B(C1=CC=C([N+]([O-])=O)C=C1)(O)O |
| CAS DataBase Reference | 24067-17-2(CAS DataBase Reference) |
Description and Uses
4-Nitrobenzeneboronic acid is an arylboronic acid building block that has been used in the synthesis of phenols.
Reagent used for
- Ligand-free palladium-catalyzed Suzuki-Miyaura cross-couplings
- Ruthenium catalyzed direct arylation of benzylic sp3 carbons of acyclic amines
- Diels-Alder or C-H activation reactions
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulations
- N-arylation of phenylurea using copper acetylacetonate catalyst
- Environmentally benign one-pot synthesis through a double arylation process
- Copper-mediated cyanations
- copper-catalyzed arylations
- Regioselective glycosylations
- Suzuki couplings followed by arylations
- X-ray absorption on rhodium-grafted hydrotalcite catalyst for heterogeneous 1,4-addition reaction of organoboron reagents to electron deficient olefins
Reagent used in Preparation of
- Combretastatin analogs as potential antitumor agents
- Human immunodeficiency virus (HIV) protease inhibitors with antiviral activities against drug-resistant viruses
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335-H302 |
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a |
| PPE | dust mask type N95 (US), Eyeshields, Gloves |
| Hazard Codes | Xn |
| Risk Statements | 22 |
| Safety Statements | 26-36/37/39-24/25 |
| WGK Germany | 3 |
| Hazard Note | Harmful |
| HS Code | 29319090 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Acute Tox. 4 Oral |