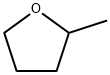
2-Methyltetrahydrofuran , ≥99%, no stabilizer , 96-47-9
Synonym(s):
2-MeTHF;2-Methyltetrahydrofuran ZerO2;Degassed and low oxygen 2-methyltetrahydrofuran;Tetrahydro-2-methylfuran;Tetrahydrosilvan
CAS NO.:96-47-9
Empirical Formula: C5H10O
Molecular Weight: 86.13
MDL number: MFCD00005367
EINECS: 202-507-4
PRODUCT Properties
| Melting point: | -136 °C |
| Boiling point: | 78-80 °C(lit.) |
| Density | 0.86 g/mL at 25 °C(lit.) |
| vapor pressure | 136 hPa (20 °C) |
| refractive index | n |
| Flash point: | 10.4 °F |
| storage temp. | Store below +30°C. |
| solubility | 150g/l |
| form | Liquid |
| color | Clear colorless |
| explosive limit | 1.2-5.7%(V) |
| Water Solubility | 15 g/100 mL (25 C) |
| FreezingPoint | -136℃ |
| BRN | 102448 |
| Dielectric constant | 6.97 |
| Stability: | Stable, but highly flammable. Incompatible with oxidizing agents, strong acids, strong bases. May form explosive peroxides in storage, so often supplied with an inhibitor added. |
| InChI | 1S/C5H10O/c1-5-3-2-4-6-5/h5H,2-4H2,1H3 |
| InChIKey | JWUJQDFVADABEY-UHFFFAOYSA-N |
| SMILES | CC1CCCO1 |
| LogP | 1.1 at 20℃ |
| CAS DataBase Reference | 96-47-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Furan, tetrahydro-2-methyl-(96-47-9) |
| EPA Substance Registry System | Furan, tetrahydro-2-methyl- (96-47-9) |
Description and Uses
2-Methyltetrahydrofuran (MeTHF or 2-MTHF) is a bio-based solvent that is recognized as the most favorable of ether solvents. The relatively high boiling point (80℃) and low melting point (-137℃) provide a broad temperature range for a myriad of processing conditions. It is a potential greener solvent alternative for organic synthesis. It shows resistance to reduction by lithium making it a promising candidate as electrolytes in lithium batteries. Its polarity and Lewis base strength is intermediate between tetrahydrofuran (THF) and diethyl ether. The ring opening reaction of 2-MTHF has been studied using acid chloride and iodide.
2-Methyltetrahydrofuran may be used as solvent for phosphatidylserine synthesis.
It may be used as an alternative solvent to:
- DMSO (dimethyl sulfoxide) or MTBE (methyl tertiary butyl ether) in the C-C bond forming reactions catalyzed by lyase enzyme.
- THF in the reaction between Grignard reagents and carbonyl compounds.
- Methylene chloride in some biphase reactions.
Safety
| Symbol(GHS) |    GHS02,GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H225-H302-H315-H318 |
| Precautionary statements | P210-P233-P280-P301+P312-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | F,Xi |
| Risk Statements | 11-19-36/37-2017/11/19 |
| Safety Statements | 16-23-39-33-26 |
| RIDADR | UN 2536 3/PG 2 |
| WGK Germany | 2 |
| RTECS | LU2800000 |
| Autoignition Temperature | 270 °C |
| TSCA | TSCA listed |
| HazardClass | 3 |
| PackingGroup | II |
| HS Code | 29321900 |
| Storage Class | 3 - Flammable liquids |
| Hazard Classifications | Acute Tox. 4 Oral Eye Dam. 1 Flam. Liq. 2 Skin Irrit. 2 |
| Toxicity | LD50 orally in Rabbit: > 300 - 2000 mg/kg LD50 dermal Rat > 2000 mg/kg |
| Limited Quantities | 1.0 L (0.3 gallon) (liquid) |
| Excepted Quantities | Max Inner Pack (30g or 30ml) and Max Outer Pack (500g or 500ml) |