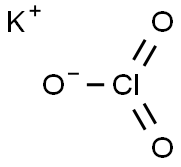Potassium chlorate , AR,99.5% , 3811-04-9
Synonym(s):
Chlorate of Potash;Potassium Chlorate
CAS NO.:3811-04-9
Empirical Formula: ClKO3
Molecular Weight: 122.549
MDL number: MFCD00011361
EINECS: 223-289-7
PRODUCT Properties
| Melting point: | 356 °C (lit.) |
| Boiling point: | 400°C |
| Density | 2,32 g/cm3 |
| bulk density | 1200-1400kg/m3 |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.440 |
| storage temp. | 2-8°C |
| solubility | water: soluble (completely 69.9 g/l at 20 °C (68 °F)) |
| form | Powder/Solid |
| color | White |
| Specific Gravity | 2.32 |
| PH | 5.6 (73g/l, H2O, 20°C) |
| Odor | Odorless |
| Water Solubility | 73 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,7620 |
| Dielectric constant | 5.1(0.0℃) |
| Stability: | Strong oxidizer - contact with combustible material may cause fire. Mixtures with combustible material may be shock-sensitive. Incompatible with organics, combustible materials, strong reducing agents. |
| Cosmetics Ingredients Functions | OXIDISING |
| InChI | 1S/ClHO3.K/c2-1(3)4;/h(H,2,3,4);/q;+1/p-1 |
| InChIKey | VKJKEPKFPUWCAS-UHFFFAOYSA-M |
| SMILES | [K+].[Cl](=O)(=O)[O-] |
| LogP | -2 at 20℃ |
| CAS DataBase Reference | 3811-04-9(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium chlorate (3811-04-9) |
Description and Uses
Potassium chlorate, is a transparent, colorless crystal or white powder. It is soluble in boiling water and decomposes at approximately 750°F (398°C), giving off oxygen gas. Potassium chlorate is a strong oxidizer and forms explosive mixtures with combustible materials, such as sugar, sulfur, and others. Potassium chlorate is incompatible with sulfuric acid, other acids, and organic material. The four-digit UN identification number is 1485. Its primary uses are as an oxidizing agent in the manufacture of explosives and matches; in pyrotechnics; and as a source of oxygen. Sodium and potassium chlorates have similar properties. Chlorites are powerful oxidizing agents. They have one less oxygen than the base-state oxysalts. They form explosive mixtures with combustible materials, and in contact with strong acids, they can release explosive chlorine dioxide gas.
Potassium Chlorate is used in micro-smoke cold fireworks containing bright bead.
Safety
| Symbol(GHS) |    GHS03,GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H271-H302+H332-H411 |
| Precautionary statements | P210-P220-P261-P273-P301+P312-P304+P340+P312 |
| Hazard Codes | O,Xn,N |
| Risk Statements | 9-20/22-51/53-52/53 |
| Safety Statements | 13-16-27-61 |
| RIDADR | UN 1485 5.1/PG 2 |
| WGK Germany | 2 |
| RTECS | FO0350000 |
| TSCA | TSCA listed |
| HS Code | 2829 19 00 |
| HazardClass | 5.1 |
| PackingGroup | II |
| Storage Class | 5.1A - Strongly oxidizing hazardous materials |
| Hazard Classifications | Acute Tox. 4 Inhalation Acute Tox. 4 Oral Aquatic Chronic 2 Ox. Sol. 1 |
| Hazardous Substances Data | 3811-04-9(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 1870 mg/kg |