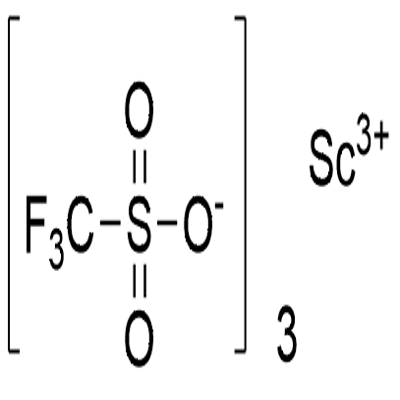
Scandium(III) triflate , 98% , 144026-79-9
Synonym(s):
Sc(OTf)3;Sc(OTf)3;Scandium tris(trifluoromethanesulfonate);Scandium(III) trifluoromethanesulfonate;Trifluoromethanesulfonic acid scandium(III) salt
| Pack Size | Price | Stock | Quantity |
| 250MG | RMB25.60 | In Stock |
|
| 1G | RMB45.60 | In Stock |
|
| 5G | RMB148.80 | In Stock |
|
| 10g | RMB311.20 | In Stock |
|
| 25G | RMB515.20 | In Stock |
|
| 100g | RMB1808.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | >300 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Water (Slightly) |
| form | Powder |
| color | White |
| Water Solubility | Soluble in water, alcohol and acetonitrile. |
| Sensitive | Hygroscopic |
| Hydrolytic Sensitivity | 6: forms irreversible hydrate |
| BRN | 8510151 |
| Stability: | hygroscopic |
| InChI | InChI=1S/3CHF3O3S.Sc/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3 |
| InChIKey | HZXJVDYQRYYYOR-UHFFFAOYSA-K |
| SMILES | C(F)(S([O-])(=O)=O)(F)F.C(F)(F)(F)S([O-])(=O)=O.C(F)(F)(F)S([O-])(=O)=O.[Sc+3] |
| CAS DataBase Reference | 144026-79-9(CAS DataBase Reference) |
Description and Uses
Scandium trifluoromethanesulfonate, commonly called Scandium(III) triflate, is a chemical compound with formula Sc(SO3CF3)3, a salt consisting of scandium cations Sc3+ and triflate SO3CF3? anions.
Scandium(III) triflate is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) triflate complex of (4′S,5′S)-2,6-bis[4′-(triisopropylsilyl)oxymethyl-5′-phenyl-1′,3′-oxazolin-2′-yl]pyridine has been employed as catalyst for the asymmetric Friedel-Crafts reaction between substituted indoles and methyl (E)-2-oxo-4-aryl-3-butenoates.
Scandium(III) trifluoromethanesulfonate is widely used as a catalyst in hydrothiolation, selective two-electron reduction of oxygen by ferrocene derivatives and vinylogous Fridel-crafts alkylation of indoles and pyrrole in water. It is involved in the Mukaiyama aldol addition and stereochemically catalyzes the radical polymerization of acrylates. It acts as a Lewis acid catalyst and used in the synthesis of bullvalone via a stabilized sulfur ylide.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338-P280a-P304+P340-P405-P501a |
| PPE | dust mask type N95 (US), Eyeshields, Gloves |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| F | 3-10 |
| Hazard Note | Irritant |
| TSCA | No |
| HS Code | 28469099 |
| Storage Class | 11 - Combustible Solids |