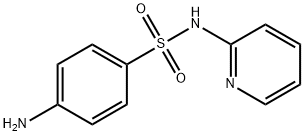
Sulfapyridine , Analysis standard , 144-83-2
Synonym(s):
N1-(Pyridin-2-yl)sulfanilamide;4-Amino-N-[2-pyridyl]benzene sulfonamide;Sulfapyridine
CAS NO.:144-83-2
Empirical Formula: C11H11N3O2S
Molecular Weight: 249.29
MDL number: MFCD00038036
EINECS: 205-642-7
| Pack Size | Price | Stock | Quantity |
| 250MG | RMB278.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 191-193°C |
| Boiling point: | 473.5±51.0 °C(Predicted) |
| Density | 1.3220 (rough estimate) |
| refractive index | 1.6740 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 8.48(H2O
t = 25
I = 0.05) (Uncertain) |
| color | White to Off-White |
| Water Solubility | <0.1 g/100 mL at 22 ºC |
| Merck | 14,8938 |
| BRN | 222065 |
| Stability: | Stable. Combustible. Protect from light. Incompatible with strong oxidizing agents. |
| Major Application | forensics and toxicology pharmaceutical (small molecule) |
| InChI | 1S/C11H11N3O2S/c12-9-4-6-10(7-5-9)17(15,16)14-11-3-1-2-8-13-11/h1-8H,12H2,(H,13,14) |
| InChIKey | GECHUMIMRBOMGK-UHFFFAOYSA-N |
| SMILES | Nc1ccc(cc1)S(=O)(=O)Nc2ccccn2 |
| CAS DataBase Reference | 144-83-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulfapyridine(144-83-2) |
| EPA Substance Registry System | Sulfapyridine (144-83-2) |
Description and Uses
Sulfapyridine is a sulfonamide antibiotic with antibacterial and anti-inflammatory activities. It is also a metabolite of sulfasalazine formed through bacterial conversion in the colon. It is active against strains of Y. enterocolitica and Salmonella (MICs = 3.1-25 and 25-100 μg/ml, respectively), as well as S. aureus (MBC = 0.8 μM). It is an inhibitor of recombinant P. carinii dihydropteroate synthetase (DHPS; IC50 = 0.18 μM). Sulfapyridine scavenges peroxyl radicals in an oxygen radical absorbance capacity (ORAC) assay. It inhibits histamine release induced by compound 48/80 from isolated rat peritoneal mast cells in a dose-dependent manner. Sulfapyridine (1 μg/kg) also inhibits compound 48/80-induced systemic allergic reaction in rats. Formulations containing sulfapyridine have previously been used in the treatment of dermatological conditions and ulcerative colitis.
antibacterial, dermatitis herpetiformis therapy
Safety
| Symbol(GHS) |  GHS08 |
| Signal word | Warning |
| Hazard statements | H361fd |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 22-24/25-36-26 |
| WGK Germany | 2 |
| RTECS | DA9625000 |
| TSCA | TSCA listed |
| HazardClass | IRRITANT |
| HS Code | 29350010 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Repr. 2 |
| Toxicity | LD50 orally in mice: 7.5 mg/g (Wien) |