A7334812
Silver nitrate solution , 0.1000mol/L(0.1N) , 7761-88-8
Synonym(s):
Argenti nitras;Lunar Caustic;Nitric acid silver(I) salt;Silver nitrate
CAS NO.:7761-88-8
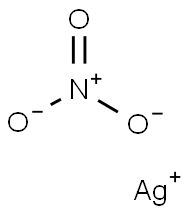
Empirical Formula: AgNO3
Molecular Weight: 169.87
MDL number: MFCD00003414
EINECS: 231-853-9
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 212 °C (dec.) (lit.) |
||||||||||||||
| Boiling point: | 444°C |
||||||||||||||
| Density | 4.35 g/mL at 25 °C(lit.) |
||||||||||||||
| bulk density | 2350kg/m3 |
||||||||||||||
| vapor density | 5.8 (vs air) |
||||||||||||||
| vapor pressure | 17.535 mm of Hg (@ 20°C) |
||||||||||||||
| Flash point: | 40 °C |
||||||||||||||
| storage temp. | 2-8°C |
||||||||||||||
| solubility | H2O: soluble |
||||||||||||||
| form | Solid |
||||||||||||||
| color | White |
||||||||||||||
| PH | 5.4-6.4 (100g/l, H2O, 20℃) |
||||||||||||||
| Odor | Odorless |
||||||||||||||
| PH Range | 7 - 9 |
||||||||||||||
| Water Solubility | 219 g/100 mL (20 ºC) |
||||||||||||||
| Sensitive | Light Sensitive |
||||||||||||||
| Merck | 14,8518 |
||||||||||||||
| crystal system | Nogata |
||||||||||||||
| Space group | Pbca |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | ACGIH: TWA 0.01 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.01 mg/m3 |
||||||||||||||
| Cosmetics Ingredients Functions | HAIR DYEING |
||||||||||||||
| InChI | 1S/Ag.NO3/c;2-1(3)4/q+1;-1 |
||||||||||||||
| InChIKey | SQGYOTSLMSWVJD-UHFFFAOYSA-N |
||||||||||||||
| SMILES | [O-][N+]([O-])=O.[Ag+] |
||||||||||||||
| CAS DataBase Reference | 7761-88-8(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | silver(I) nitrate(7761-88-8) |
||||||||||||||
| EPA Substance Registry System | Silver nitrate (7761-88-8) |
Description and Uses
The basis of nearly all photographic silver halides with the exception of the daguerreotype process, silver nitrate is a heavy white crystal made by dissolving elemental silver in nitric acid followed by evaporation. It is soluble in water, ether, and glycerin. Silver nitrate is not sensitive to light, but when combined with an organic material, a halogen, or a halide it will reduce back to a metallic state when exposed to light.
Safety
| Symbol(GHS) |    GHS03,GHS05,GHS09 |
| Signal word | Danger |
| Hazard statements | H272-H290-H314-H410 |
| Precautionary statements | P210-P260-P273-P280-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | C,O,N,Xi |
| Risk Statements | 34-50/53-8-36/38-51/53-52/53-35-10-40-20/22-22 |
| Safety Statements | 26-45-60-61-36/37/39-27-57-37 |
| RIDADR | UN 1493 5.1/PG 2 |
| WGK Germany | 3 |
| RTECS | VW4725000 |
| F | 8 |
| TSCA | TSCA listed |
| HazardClass | 5.1 |
| PackingGroup | II |
| HS Code | 28432100 |
| Storage Class | 5.1B - Oxidizing hazardous materials |
| Hazard Classifications | Aquatic Acute 1 Aquatic Chronic 1 Eye Dam. 1 Met. Corr. 1 Ox. Sol. 2 Repr. 1B Skin Corr. 1A |
| Hazardous Substances Data | 7761-88-8(Hazardous Substances Data) |
| Toxicity | LD50 oral in rat: 1173mg/kg |
| Limited Quantities | 1.0 L (0.3 gallon) (liquid) or 1.0 kg (2.2 lbs) (solid) |
| Excepted Quantities | Max Inner Pack (30g or 30ml) and Max Outer Pack (500g or 500ml) |