PRODUCT Properties
| Boiling point: | 152-156 °C |
| Density | 1.0630 g/cm3 |
| storage temp. | Amber Vial, -20°C Freezer |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Oil |
| color | Colourless |
| Odor | at 100.00 %. spice flower |
| Odor Type | spicy |
| Stability: | Light Sensitive |
| InChI | InChI=1S/C12H16O3/c1-5-6-9-7-10(13-2)12(15-4)11(8-9)14-3/h5,7-8H,1,6H2,2-4H3 |
| InChIKey | BPLQKQKXWHCZSS-UHFFFAOYSA-N |
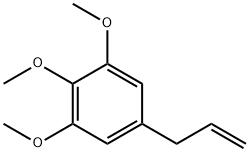
| SMILES | C1(OC)=CC(CC=C)=CC(OC)=C1OC |
| LogP | 2.298 (est) |
| NIST Chemistry Reference | Benzene, 1,2,3-trimethoxy-5-(2-propenyl)-(487-11-6) |
Description and Uses
Elemicin is a trioxygenated phenylpropane that has been found in A. dracunculus. It is active against S. aureus, B. subtilis, and C. albicans (MICs = 600, 2,500, and 1,000 mg/L, respectively) but not E. coli, K. pneumoniae, P. aeruginosa (MICs = >8,000 mg/L for all), or L. monocytogenes (MIC = >3,000 mg/L). Elemicin is toxic to mice following metabolic activation to 1’-hydroxyelemicin by the cytochrome P450 (CYP) isoforms CYP1A1 and CYP1A2. It increases plasma and hepatic triglyceride levels, decreases stearoyl-CoA desaturase 1 (Scd1) expression, and induces hepatomegaly in mice when administered at a dose of 500 mg/kg per day for three weeks.
A constituent of the essential oil of nutmeg and is responsible for the psychoactive effects of nutmeg. Also a minor constituent of the oleoresin and essential oil of Manila elemi (Canarium luzonicum). Exhibits anticholinergic-like effects in humans.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
| HS Code | 2909309090 |