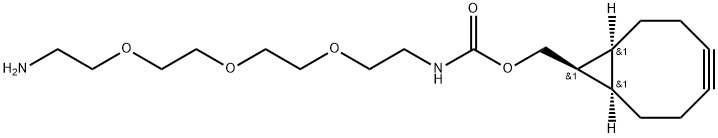
BD8886755
rel-((1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-yl)methyl(4-nitrophenyl)carbonate , 95% , 1263166-91-1
| Pack Size | Price | Stock | Quantity |
| 25mg | RMB794.40 | In Stock |
|
| 50mg | RMB1350.40 | In Stock |
|
| 100mg | RMB2294.40 | In Stock |
|
| 250mg | RMB4129.60 | In Stock |
|
| 1g | RMB11149.60 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Boiling point: | 475.7±37.0 °C(Predicted) |
| Density | 1.32±0.1 g/cm3(Predicted) |
| storage temp. | Storage temp. -20°C |
| solubility | DMSO : 100 mg/mL (317.14 mM; Need ultrasonic) |
| form | Solid |
| color | White to off-white |
| InChI | InChI=1/C17H17NO5/c19-17(23-13-9-7-12(8-10-13)18(20)21)22-11-16-14-5-3-1-2-4-6-15(14)16/h7-10,14-16H,3-6,11H2/t14-,15+,16- |
| InChIKey | QXNXOXMDBLHIDB-MUJYYYPQNA-N |
| SMILES | C([C@H]1[C@@]2([H])CCC#CCC[C@@]12[H])OC(=O)OC1C=CC(N(=O)=O)=CC=1 |&1:1,2,10,r| |
Description and Uses
endo-BCN-PNP-carbonate is a very reactive compound which can easily react with amine-containing molecules in organic solvents. PNP is a good leaving group. The BCN is used for copper-free Click Chemistry reactions.
endo-BCN-O-PNB is an alkyl/ether-based PROTAC linker that can be used in the synthesis of PROTACs[1]. endo-BCN-O-PNB is a click chemistry reagent, it contains a BCN group that can undergo strain-promoted alkyne-azide cycloaddition (SPAAC) with molecules containing Azide groups.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |

![rel-((1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-yl)methyl(4-nitrophenyl)carbonate](https://img.chemicalbook.com/CAS/20211123/GIF/1263166-91-1.gif)
![exo-Bicyclo[6.1.0]non-4-yn-9-ylmethanol](https://img.chemicalbook.com/CAS/20180527/GIF/1263291-41-3.gif)

![rel-((1R,8S,9r)-Bicyclo[6.1.0]non-4-yn-9-yl)methyl(4-nitrophenyl)carbonate](https://img.chemicalbook.com/CAS/20210111/GIF/1380006-72-3.gif)