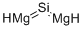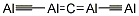Magnesium silicide , 99.99 , 22831-39-6
Synonym(s):
Dimagnesium silicide
CAS NO.:22831-39-6
Empirical Formula: Mg2Si
Molecular Weight: 76.7
MDL number: MFCD00016202
EINECS: 245-254-5
PRODUCT Properties
| Melting point: | 1102 °C |
| Density | 1,94 g/cm3 |
| solubility | reacts with H2O |
| form | Powder |
| Specific Gravity | 2. |
| color | Blue |
| Water Solubility | Insoluble in water and denser than water. |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| Crystal Structure | Cubic, Antifluorite Structure - Space Group Fm3m |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,5688 |
| Stability: | Spontaneously flammable - mixtures with air are explosive. Reacts with water to form toxic vapours. |
| InChI | 1S/2Mg.Si |
| InChIKey | YTHCQFKNFVSQBC-UHFFFAOYSA-N |
| SMILES | [Mg]=[Si]=[Mg] |
| EPA Substance Registry System | Magnesium silicide (Mg2Si) (22831-39-6) |
Description and Uses
Magnesium silicide has the molecular formula of
Mg2Si and the molecular weight of 76.6955 g/mol. Its density is 1.998 g/cc
and its melting point is 1102°C. It is a black powder.
Mg2Si crystallizes in a cubic CaF2 (fluorspar)-type
lattice with a = 4.49 ? . The centers of this lattice are analogous
to that of diamond. Each Si atom forms four covalent
sp3 bonds. The Mg subshells are only half-filled.
There is no bonding between the two Mg atom sites.
The distance between the Mg and Si atoms is 2.77 ? .
Bonding in this compound is essentially covalent.
Magnesium silicide is used to produce alloys such as aluminum alloys. It is also used in food, beverages, as a flavor enhancer and as an intermediate in chemical research.
Safety
| Symbol(GHS) |  GHS02 |
| Signal word | Danger |
| Hazard statements | H261 |
| Precautionary statements | P231+P232-P422 |
| Hazard Codes | F |
| Risk Statements | 14-14/15 |
| Safety Statements | 7/8-43 |
| RIDADR | UN 2624 4.3/PG 2 |
| WGK Germany | 3 |
| TSCA | TSCA listed |
| HazardClass | 4.3 |
| PackingGroup | II |
| Storage Class | 4.3 - Hazardous materials which set free flammable gases upon contact with water |
| Hazard Classifications | Water-react 2 |