Calcium nitride , 99 , 12013-82-0
Synonym(s):
Tricalcium dinitride
CAS NO.:12013-82-0
Empirical Formula: Ca3N2
Molecular Weight: 148.25
MDL number: MFCD00015985
EINECS: 234-592-9
| Pack Size | Price | Stock | Quantity |
| 5g | RMB564.80 | In Stock |
|
| 50g | RMB3572.00 | In Stock |
|
| 250g | RMB17700.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 1195°C |
| Boiling point: | 1195 °C |
| Density | 2.63 g/mL at 25 °C(lit.) |
| solubility | soluble in H2O, acid solutions; insoluble in ethanol |
| form | Pieces |
| Specific Gravity | 2.63 |
| color | Brown |
| Water Solubility | Insoluble in water. |
| Sensitive | Moisture Sensitive |
| InChI | 1S/3Ca.2N |
| InChIKey | DVJMVZQXJNJSJR-UHFFFAOYSA-N |
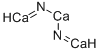
| SMILES | [Ca]=N[Ca]N=[Ca] |
| CAS DataBase Reference | 12013-82-0(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium nitride (Ca3N2) (12013-82-0) |
Description and Uses
Calcium nitride has the formula of Ca3N2 and the
molecular weight of grams per mole. It can be prepared
by the reactionofCametal inapure streamof nitrogen gas:
3Ca + 2N2 ? Ca3N2
A second method is the decomposition of an amide:
3Ca(NH2)2 + heat ? Ca3N2 + 4NH3
This must be accomplished in an inert atmosphere
(not nitrogen gas). It is a reddish-brown powder. Its
CAS number is 12013-82-0 and its density is 2.76 g/
cm3. It melts at 1195 °C (2183 F).α-Calcium nitride
is the commonly encountered form. It has an antibixbyite
structure similar to Mn2O3, except that the
positions of the ions are reversed: calcium (Ca2+)
ions take the oxide (O2-) positions and nitride ions
(N3-) that of the manganese (Mn3+). If Ca metal is
burned in air, it forms the oxide, CaO, and the
nitride, Ca3N2. The latter reacts with atmospheric
moisture to produce the hydroxide of calcium and
ammonia:
Ca3N2 + 6H2O ? 3Ca(OH)2 + 2NH3
Calcium nitride is used as a nitride source in metathesis reactions to produce complex nitrides as well as SiAlON-related optical materials.
Safety
| Symbol(GHS) |   GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | H260-H314 |
| Precautionary statements | P231+P232-P260-P280-P301+P330+P331-P303+P361+P353-P305+P351+P338 |
| PPE | Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges |
| Hazard Codes | F,C |
| Risk Statements | 11-29-34 |
| Safety Statements | 16-22-26-36/37/39-45 |
| RIDADR | UN 3208 4.3/PG 1 |
| WGK Germany | 3 |
| TSCA | TSCA listed |
| HazardClass | 4.1 |
| PackingGroup | II |
| Storage Class | 4.3 - Hazardous materials which set free flammable gases upon contact with water |
| Hazard Classifications | Skin Corr. 1B Water-react 1 |