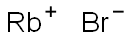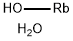Rubidium bromide , 99.8 , 7789-39-1
Synonym(s):
Rubidium monobromide
CAS NO.:7789-39-1
Empirical Formula: BrRb
Molecular Weight: 165.37
MDL number: MFCD00011186
EINECS: 232-162-5
| Pack Size | Price | Stock | Quantity |
| 5g | RMB473.60 | In Stock |
|
| 25g | RMB1591.20 | In Stock |
|
| 100g | RMB5616.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 693 °C |
| Boiling point: | 1340 °C |
| Density | 3.35 g/mL at 25 °C (lit.) |
| refractive index | 1.5530 |
| Flash point: | 1340°C |
| form | crystal |
| Specific Gravity | 3.35 |
| color | white |
| Water Solubility | Soluble in water |
| Sensitive | Hygroscopic |
| Merck | 14,8283 |
| Dielectric constant | 4.8499999999999996 |
| Stability: | Stable. Incompatible with strong acids. |
| InChI | 1S/BrH.Rb/h1H;/q;+1/p-1 |
| InChIKey | JAAGVIUFBAHDMA-UHFFFAOYSA-M |
| SMILES | [Br-].[Rb+] |
| CAS DataBase Reference | 7789-39-1(CAS DataBase Reference) |
| EPA Substance Registry System | Rubidium bromide (RbBr) (7789-39-1) |
Description and Uses
Rubidium bromide (RbBr) is a hygroscopic material, which is generally similar to the other rubidium halides.
Rubidium bromide is the bromide of rubidium. It has a NaCl crystal structure, with a lattice constant of 685 picometres.
There are several methods for synthesising rubidium bromide. One involves reacting rubidium hydroxide with hydrobromic acid:
RbOH + HBr → RbBr + H2O
Another method is to neutralize rubidium carbonate with hydrobromic acid:
Rb2CO3 + 2 HBr → 2 RbBr + H2O + CO2
Rubidium metal would react directly with bromine to form RbBr, but this is not a sensible production method, since rubidium metal is substantially more expensive than the carbonate or hydroxide; moreover, the reaction would be explosive.
Rubidium bromide is used in infrared optics.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P280-P302+P352-P362+P364 |
| PPE | Eyeshields, Gloves, type N95 (US) |
| Safety Statements | 22-24/25 |
| WGK Germany | 3 |
| TSCA | TSCA listed |
| HS Code | 2827590000 |
| Storage Class | 11 - Combustible Solids |