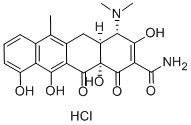
AnhydrotetracyclineHydrochloride , 98% , 13803-65-1
Synonym(s):
(4S,4aS,12aS)-4-(Dimethylamino)-1,4,4a,5,12,12a-hexahydro-3,10,11,12a-tetrahydroxy-6-methyl-1,12-dioxo-2-naphthacenecarboxamide monohydrochloride
CAS NO.:13803-65-1
Empirical Formula: C22H23ClN2O7
Molecular Weight: 462.88
MDL number: MFCD00151453
| Pack Size | Price | Stock | Quantity |
| 25mg | RMB659.20 | In Stock |
|
| 100mg | RMB1614.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 223 °C |
| RTECS | QI7860000 |
| storage temp. | Refrigerator |
| solubility | Soluble in ethanol, DMSO, or DMF |
| form | Powder |
| color | Yellow to bright yellow |
| Sensitive | Moisture Sensitive |
| BRN | 4228856 |
| Stability: | Hygroscopic |
| Major Application | clinical testing |
| InChIKey | XBSQEFHDCDFNJU-WPJNXPDPSA-N |
| SMILES | Cl[H].[H][C@@]12Cc3c(C)c4cccc(O)c4c(O)c3C(=O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@H]2N(C)C |
| EPA Substance Registry System | 2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,12,12a-hexahydro-3,10,11,12a-tetrahydroxy-6-methyl-1,12-dioxo-, hydrochloride (1:1), (4S,4aS,12aS)- (13803-65-1) |
Description and Uses
The tetracycline repressor (TetR) is a transcriptional regulator which normally binds tightly to its palindromic tetO operator DNA, blocking gene expression. Tet causes the repressor to dissociate from the DNA, allowing transcription to occur. A novel reverse TetR (revTetR) requires tetracycline as a co-
Anhydrotetracycline is a degradation product of tetracycline, formed by dehydration at the C6 position under acidic conditions to aromatise the B ring. Anhydrotetracycline is an important standard for monitoring tetracycline stability. Although the degradation is associated with a loss of antibiotic activity, anhydrotetracycline is considered biologically active and is thought responsible for aspects of tetracycline toxicity.
Safety
| Symbol(GHS) |   GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319-H361 |
| Precautionary statements | P201-P202-P264b-P280i-P305+P351+P338-P308+P313-P403-P501c |
| PPE | Eyeshields, Gloves, type N95 (US) |
| Hazard Codes | Xn |
| Risk Statements | 63-36 |
| Safety Statements | 36/37/39-26 |
| WGK Germany | 3 |
| HS Code | 29413020 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 4 Oral Repr. 1B |