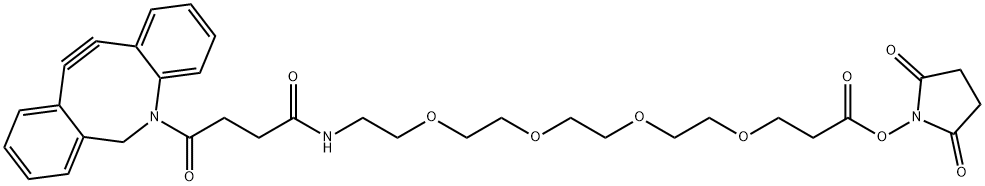
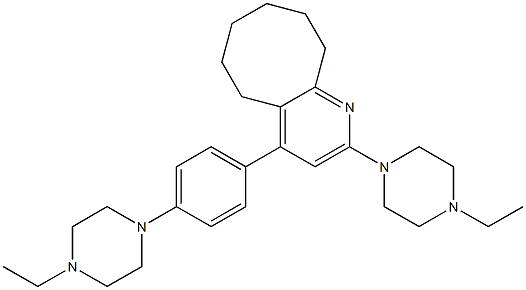
DBCO-PEG4-NHSEster , 95% , 1427004-19-0
Synonym(s):
DBCO-PEG4-NHS ester;DBCO-PEG4-SE;DBCO-PEG4-succinimidyl ester
CAS NO.:1427004-19-0
Empirical Formula: C34H39N3O10
Molecular Weight: 649.69
MDL number: MFCD26793797
| Pack Size | Price | Stock | Quantity |
| 50mg | RMB4160.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO, DCM, DMF |
| pka | 15.14±0.46(Predicted) |
| form | gel or oil |
| color | Colourless to yellow |
| InChIKey | RRCXYKNJTKJNTD-UHFFFAOYSA-N |
| SMILES | C(ON1C(=O)CCC1=O)(=O)CCOCCOCCOCCOCCNC(=O)CCC(N1CC2=CC=CC=C2C#CC2=CC=CC=C12)=O |
Description and Uses
DBCO-PEG4-NHS Ester is a click chemistry PEG reagent containing NHS ester that is able to react specifically and efficiently with primary amines (e.g. the side chain of lysine residues or aminosilane-coated surfaces) at neutral or slightly basic condition to form a covalent bond. The hydrophilic PEG spacer arm improves water solubility and provides a long and flexible connection that minimizes steric hindrance involved with ligation. DBCO is commonly used for copper-free Click Chemistry reactions.
Succinimidyl ester (NHS, amine reactive) functionalized cyclooctyne derivative for incorporation of the cyclooctyne moiety into amine containing compounds or biomolecules. Cyclooctynes are useful in strain-promoted copper-free click chemistry cycloaddition reactions. This dibenzocyclooctyne will react with azide functionalized compounds or biomolecules without the need for a Cu(I) catalyst to result in a stable triazole linkage.
Applications Include:
- Protein-peptide conjugates
- Antibody-enzyme or antibody-drug conjugates
- Protein or peptide-oligonucleotide conjugates
- Surface modification
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| WGK Germany | 3 |
| HS Code | 2942000090 |