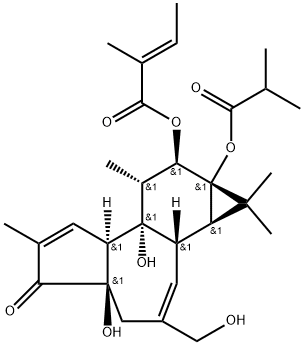
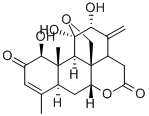
Ailanthone , ≥98%(HPLC) , 981-15-7
Synonym(s):
Δ13-Dehydrochaparrinone;(1β,11β,12α)-11,20-Epoxy-1,11,12-trihydroxypicrasa-3,13(21)-diene-2,16-dione;13,21-Didehydrochaparrinone
| Pack Size | Price | Stock | Quantity |
| 10mg | RMB5193.60 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 235-237 °C |
| Boiling point: | 641.0±55.0 °C(Predicted) |
| Density | 1.47 |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO:100.0(Max Conc. mg/mL);265.67(Max Conc. mM) |
| form | A crystalline solid |
| pka | 11.85±0.70(Predicted) |
| color | White to off-white |
| InChIKey | WBBVXGHSWZIJST-BIKIFPNSNA-N |
| SMILES | [C@]123CO[C@@]4(O)[C@H](O)C(=C)C1CC(=O)O[C@]2([H])C[C@@]1([H])C(C)=CC(=O)[C@@H](O)[C@]1(C)[C@]34[H] |&1:0,3,5,14,17,24,26,28,r| |
| LogP | -0.760 (est) |
Description and Uses
Ailanthone is a quassinoid that has been found in Ailanthus and has diverse biological activities. It is active against the P. falciparum strains HB-3 and Dd-2 in vitro (IC50s = 0.003 and 0.037 μg/ml, respectively). Ailanthone is phytotoxic, inhibiting radish seed germination by 88% when used at a concentration of 1 mM. It inhibits dihydrotestosterone-induced androgen receptor transcriptional activity (IC50 = 69 nM in a reporter assay), as well as the growth and colony formation of LNCaP and 22RV1 androgen receptor-expressing cells, but not androgen receptor-negative PC3 and DU145 cells, when used at a concentration of 0.1 μM. Ailanthone (2 mg/kg) reduces tumor volume in 22Rv1, LNCaP, and VCaP castration-resistant prostate cancer (CRPC) mouse xenograft models.
Ailanthone has potent antineoplastic activity against anti-hepatocellular carcinoma (HCC) and has been shown to inhibit Huh7 cancer cell growth through cell cycle arrest and apoptosis in vitro and in vivo.
Safety
| Symbol(GHS) |  GHS06 |
| Signal word | Danger |
| Hazard statements | H300 |
| Precautionary statements | P301+P330+P331+P310 |

![(2S,4aS,6aR,7S,9R,11aS,11bS)-7-Hydroxy-2-(((2R,3R,4R,5R,6R)-6-(hydroxymethyl)-3-((3-methylbutanoyl)oxy)-4,5-bis(sulfooxy)tetrahydro-2H-pyran-2-yl)oxy)-11b-methyl-8-methylenedodecahydro-6a,9-methanocyclohepta[a]naphthalene-4,4(4aH)-dicarboxylicaciddipotassiumsalt](https://img.chemicalbook.com/CAS/GIF/33286-30-5.gif)