S850078
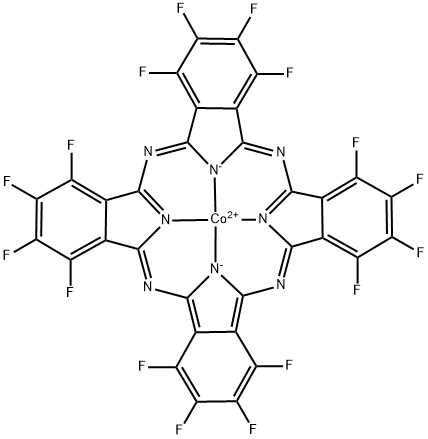
Cobalt(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine , Dyecontent95 % , 52629-20-6
Synonym(s):
(Hexadecafluorophthalocyaninato)cobalt;Cobalt hexadecafluorophthalocyanine
| Pack Size | Price | Stock | Quantity |
| 1g | RMB998.92 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | >300 °C (lit.) |
| storage temp. | 2-8°C, stored under nitrogen |
| form | powder |
| Appearance | Brown to black Solid |
| λmax | 678 nm |
| InChIKey | OFILAZNRYPZFNX-UHFFFAOYSA-N |
| SMILES | FC1C(=C(F)C(F)=C2C3=NC4C5=C(C(F)=C(F)C(F)=C5C5=NC6=C7C(=C(F)C(F)=C(F)C7=C7N=C8C9=C(C(F)=C(F)C(F)=C9C9=N8[Co+2](N=45)([N-]76)[N-]3C(=N9)C=12)F)F)F)F |
Description and Uses
The graphite electrode surface onto which Cobalt(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine (CoPcF16; CoIIHFPC)is adsorbed displays a strong electrocatalytic activity toward O2 reduction. CoIIHFPC instead of CoPc(NH2)4, as the catalyst for electrochemical reduction of O2 because CoPcF16 contains electro-withdrawing groups, and thus, has a higher catalytic activity and is more stable for O2 reduction compared with CoPc(NH2)4. The OH- concentration has a very strong effect on the reduction process. Higher OH- concentration could stabilize O-2 ion[1-3].
Safety
| Symbol(GHS) |   GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302+H312+H332-H315-H319-H351 |
| Precautionary statements | P501-P261-P270-P202-P201-P271-P264-P280-P308+P313-P337+P313-P305+P351+P338-P362+P364-P332+P313-P301+P312+P330-P302+P352+P312-P304+P340+P312-P405 |
| WGK Germany | 3 |
| Storage Class | 11 - Combustible Solids |