PRODUCT Properties
| Melting point: | decomposes [CRC10] |
| Density | 2.2 g/mL at 25 °C (lit.) |
| storage temp. | Store at room temperature, keep dry and cool |
| form | Powder |
| color | White |
| Water Solubility | g/L solution H2O: 0.0069 (25°C), 0.0142 (95°C); solid phase, CaC2O4 ·H2O [KRU93]; soluble dilute HCl, HNO3 [HAW93] |
| Merck | 13,1690 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
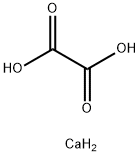
| InChI | 1S/C2H2O4.Ca/c3-1(4)2(5)6;/h(H,3,4)(H,5,6);/q;+2/p-2 |
| InChIKey | QXDMQSPYEZFLGF-UHFFFAOYSA-L |
| SMILES | O=C1O[Ca]OC1=O |
| CAS DataBase Reference | 563-72-4(CAS DataBase Reference) |
| EPA Substance Registry System | Ethanedioic acid, calcium salt (1:1) (563-72-4) |
Description and Uses
Calcium oxalate (in archaic terminology, oxalate of lime) is a chemical compound that forms envelope-shaped crystals, known in plants as raphides. A major constituent of human kidney stones, the chemical is also found in beerstone, a scale that forms on containers used in breweries. Its chemical formula is CaC2O4 or Ca (COO)2.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302+H312 |
| Precautionary statements | P264-P270-P280-P301+P312-P302+P352+P312-P362+P364 |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22-36/37/38-21/22 |
| Safety Statements | 26-37/39-24/25 |
| WGK Germany | 3 |
| TSCA | TSCA listed |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Acute Tox. 4 Dermal Acute Tox. 4 Oral |