A0740912
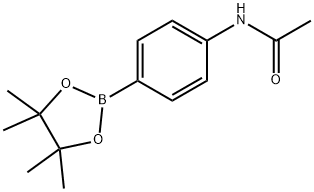
4-Aminophenylboronic acid pinacol ester , 98% , 214360-73-3
Synonym(s):
2-(4-Aminophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)aniline;4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzeneamine;4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenylamine;4-Aminophenylboronic acid, pinacol cyclic ester
CAS NO.:214360-73-3
Empirical Formula: C12H18BNO2
Molecular Weight: 219.09
MDL number: MFCD02258965
EINECS: 629-061-7
| Pack Size | Price | Stock | Quantity |
| 1G | RMB24.00 | In Stock |
|
| 5G | RMB84.80 | In Stock |
|
| 25G | RMB325.60 | In Stock |
|
| 100G | RMB943.20 | In Stock |
|
| 500g | RMB3917.60 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 165-169 °C (lit.) |
| Boiling point: | 340.0±25.0 °C(Predicted) |
| Density | 1.05±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Chloroform |
| form | Crystalline Powder |
| pka | 4.24±0.10(Predicted) |
| color | Almost white to light beige |
| Water Solubility | Insoluble |
| InChI | InChI=1S/C12H18BNO2/c1-11(2)12(3,4)16-13(15-11)9-5-7-10(14)8-6-9/h5-8H,14H2,1-4H3 |
| InChIKey | ZANPJXNYBVVNSD-UHFFFAOYSA-N |
| SMILES | C1(N)=CC=C(B2OC(C)(C)C(C)(C)O2)C=C1 |
| CAS DataBase Reference | 214360-73-3(CAS DataBase Reference) |
Description and Uses
4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)aniline is an important organic reagent with an appearance ranging from white to yellow to orange powder to crystal. This compound can be used as a chemical reaction reagent or for preparing fluorescent probes.
4-Aminophenylboronic acid pinacol ester can be used as a reagent for:
- The preparation of substituted 3-phenyl-4H-1-benzopyran-4-ones by reacting with iodochromones via Pd catalyzed Suzuki-Miyaura cross-coupling reaction.
- Mercury(II) detection by fluorometry with new fluorogenic indicators based on through-bond energy transfer from pentaquinone to rhodamine.
- Rhodium-catalyzed amination reactions.
- Palladium-catalyzed Suzuki cross-coupling to synthesize potential antitubercular and antimicrobial compounds.
It can also be used to prepare:
- Hexaphenylbenzene derivatives as a potential bioprobe and multichannel keypad system.
- Pyromellitic diimide-based polymer as matrix for solution-processable n-channel field-effect transistors.
- Alternating copolymers of oligoarylenes and naphthalene bisimides as low band-gap semiconductors with electrochemical and spectroelectrochemical behavior.
- γ-secretase modulators in the treatment of amyloid β formation.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 |
| target organs | Respiratory system |
| PPE | dust mask type N95 (US), Eyeshields, Gloves |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36-37/39 |
| WGK Germany | 3 |
| TSCA | No |
| HazardClass | IRRITANT |
| HS Code | 29319090 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |

![tert-butyl N-[4-(tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]carbamate](https://img.chemicalbook.com/CAS/GIF/330793-01-6.gif)