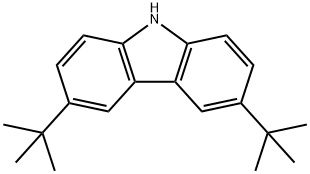
3,6-Di-tert-butylcarbazole , 98% , 37500-95-1
Synonym(s):
3,6-Di-tert-butyl-9H-carbazole
| Pack Size | Price | Stock | Quantity |
| 1G | RMB23.20 | In Stock |
|
| 5G | RMB54.40 | In Stock |
|
| 25g | RMB156.80 | In Stock |
|
| 100g | RMB574.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 233-235℃ |
| Boiling point: | 424.2±14.0 °C(Predicted) |
| Density | 1.037 |
| storage temp. | Sealed in dry,Room Temperature |
| form | powder |
| pka | 17.70±0.30(Predicted) |
| color | White to Almost white |
| InChI | InChI=1S/C20H25N/c1-19(2,3)13-7-9-17-15(11-13)16-12-14(20(4,5)6)8-10-18(16)21-17/h7-12,21H,1-6H3 |
| InChIKey | OYFFSPILVQLRQA-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=C(C(C)(C)C)C=C2)C2=C1C=CC(C(C)(C)C)=C2 |
Description and Uses
3,6-Di-tert-butylcarbazole (DtBuCz) is an electron-donating 9h-carbazole derivative with tert-butyl groups at the 3,6 positions. The bulky tert-butyl group can disrupt the aggregation of dimers or excimers that would otherwise form through π-π interactions by increasing the distance between the carbazole rings, leading to loose molecular stacking in thin films. Carbazoles are generally electrochemically active due to the dimerization and polymerization of the corresponding radical cations at their 3,6 positions. Substitution with tert-butyl groups at the 3,6 positions can also inhibit this reaction. 3,6-Di-tert-butylcarbazole has also been incorporated into semiconducting small molecules for OLED applications to achieve higher glass transition temperatures. The introduction of the tert-butyl unit also effectively separates the molecules, reducing the chance of triplet polaron annihilation and significantly improving device efficiency and stability.
3,6-Di-tert-butylcarbazole is mainly used as a monomeric precursor in the syntheses of new carbazole based materials which consist of ethynylphenyl. These materials include 9-(4-bromophenyl)-3,6-di-tert-butylcarbazol and 2-(4-(2-(4-(3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl)ethynyl)benzylidene)malononitrile (PBM) which can be further be used in organic light emitting diodes (OLEDs) and optical switching devices.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P264-P270-P301+P312-P302+P352-P305+P351+P338 |
| target organs | Respiratory system |
| Hazard Codes | Xn |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26 |
| WGK Germany | 3 |
| HS Code | 29339900 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Acute Tox. 4 Oral Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |