A3365912
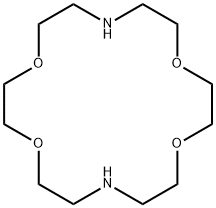
4,13-Diaza-18-crown 6-Ether , ≥98.0% , 23978-55-4
Synonym(s):
1,10-Diaza-18-crown-6;1,7,10,16-Tetraoxa-4,13-diazacyclooctadecane;Kryptofix 22
CAS NO.:23978-55-4
Empirical Formula: C12H26N2O4
Molecular Weight: 262.35
MDL number: MFCD00005112
EINECS: 245-965-0
| Pack Size | Price | Stock | Quantity |
| 250MG | RMB318.40 | In Stock |
|
| 1G | RMB784.00 | In Stock |
|
| 5G | RMB3184.00 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 111-114 °C(lit.) |
| Boiling point: | 405.6°C (rough estimate) |
| Density | 1.0807 (rough estimate) |
| refractive index | 1.4560 (estimate) |
| storage temp. | Store below +30°C. |
| pka | 9.00±0.20(Predicted) |
| form | Shiny Crystalline Powder |
| color | Off-white |
| Water Solubility | Soluble in water. |
| BRN | 609764 |
| InChI | InChI=1S/C12H26N2O4/c1-5-15-9-10-17-7-3-14-4-8-18-12-11-16-6-2-13-1/h13-14H,1-12H2 |
| InChIKey | NLMDJJTUQPXZFG-UHFFFAOYSA-N |
| SMILES | O1CCNCCOCCOCCNCCOCC1 |
| CAS DataBase Reference | 23978-55-4(CAS DataBase Reference) |
Description and Uses
1,4,10,13-Tetraoxa-7,16-diazacyclooctadecane is a crown ether, which can be used:
- In the spectroscopic studies of its complex-forming reaction with iodine.
- To prepare the ligand 7,16-bis(5-t-butyl-2-hydroxybenzyl)-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane.
- As a ligand in the study of zirconium facilitated hydrolysis of a dipeptide at neutral pH.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 |
| target organs | Respiratory system |
| PPE | dust mask type N95 (US), Eyeshields, Gloves |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-37/39-37 |
| WGK Germany | 2 |
| RTECS | HM1650000 |
| F | 10 |
| HS Code | 2934 99 90 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |
| Toxicity | LD50 orally in Rabbit: 3400 mg/kg |

![4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane](https://img.chemicalbook.com/CAS/GIF/23978-09-8.gif)
![4,7,13,16,21-Pentaoxa-1,10-diazabicyclo[8.8.5]tricosane](https://img.chemicalbook.com/CAS/GIF/31364-42-8.gif)