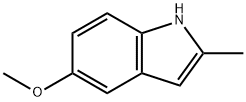
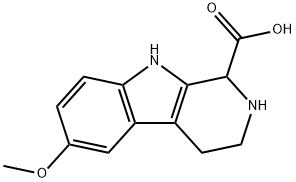
5-Methoxy-2-methylindole , 98% , 1076-74-0
Synonym(s):
NSC 63817
CAS NO.:1076-74-0
Empirical Formula: C10H11NO
Molecular Weight: 161.2
MDL number: MFCD00005620
EINECS: 214-066-5
| Pack Size | Price | Stock | Quantity |
| 1G | RMB99.20 | In Stock |
|
| 5G | RMB433.60 | In Stock |
|
| 25G | RMB1759.20 | In Stock |
|
| 100G | RMB4679.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 86-88 °C (lit.) |
| Boiling point: | 145 °C / 1.5mmHg |
| Density | 1.0840 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Soluble in methanol (very faint turbidity.) |
| pka | 17.28±0.30(Predicted) |
| form | Crystalline Powder or Crystals |
| color | White to light beige |
| InChI | InChI=1S/C10H11NO/c1-7-5-8-6-9(12-2)3-4-10(8)11-7/h3-6,11H,1-2H3 |
| InChIKey | VSWGLJOQFUMFOQ-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=C(OC)C=C2)C=C1C |
| CAS DataBase Reference | 1076-74-0(CAS DataBase Reference) |
Description and Uses
5-methoxy-2-methylindole is a useful organic raw material. It can be used as the reactant in the preparation of indolylquinoxalines by condensation reactions, in preparation of alkylindoles via Ir-catalyzed reductive alkylation, in arylation reactions in the presence of a palladium acetate catalyst, in enantioselective Friedel-Crafts alkylation, as well as in stereo selective synthesis of cyclopentaindolones via stereoselective [3+2] cyclopentannulation. It is also employed in inhibiting the chlorinating activity of myeloperoxidase (MPO) and in the metabolic synthesis of acrylacetic acid anti-inflammatory synthesis.
It is employed as a reactant in preparation of indolylquinoxalines by condensation reactions, reactant in preparation of alkylindoles via Ir-catalyzed reductive alkylation, reactant in arylation reactions using a palladium acetate catalyst, reactant in enantioselective Friedel-Crafts alkylation and reactant in stereoselective synthesis of cyclopentaindolones via stereoselective [3+2] cyclopentannulation. As an indole derivative shown to be an effective inhibitor of the chlorinating activity of myeloperoxidase (MPO). Used in the metabolic synthesis of arylacetic acid anti-inflammatory synthesis.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H332-H335 |
| Precautionary statements | P261-P280-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 37/39-26 |
| WGK Germany | 3 |
| HazardClass | IRRITANT |
| HS Code | 29339900 |
| Storage Class | 11 - Combustible Solids |