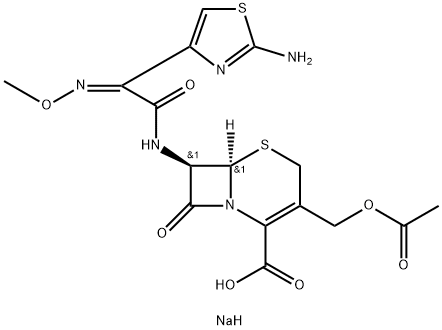
Cefotaximesodiumsalt , 10mMinDMSO , 64485-93-4
Synonym(s):
Cefotaxim sodium salt;Cefotaxime sodium salt
CAS NO.:64485-93-4
Empirical Formula: C16H16N5NaO7S2
Molecular Weight: 477.45
MDL number: MFCD00079073
EINECS: 264-915-9
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB159.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 162-163 C |
| alpha | D20 +55±2° (c = 0.8 in water) |
| refractive index | 61 ° (C=1, H2O) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O: aqueous solutions of pH 4.3-6.2 are stable up to 3 weeks at 2-8 °C.soluble |
| form | powder |
| color | white to yellow |
| PH | pH(100g/l, 25℃) : 4.5~6.5 |
| optical activity | [α]/D +58 to +64°, c =1 in H2O |
| Water Solubility | Soluble in water. |
| Merck | 14,1933 |
| BRN | 5711411 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | ACGQSCNTUWPWNB-RRVHIMMXNA-N |
| SMILES | C(C1=C(CS[C@]2([H])[C@H](NC(=O)/C(/C3=CSC(N)=N3)=N\OC)C(=O)N12)COC(=O)C)(=O)O.[NaH] |&1:5,7,r| |
Description and Uses
Cefotaxime was synthesized by Hoechst and Roussel-Uclaf in 1977. It was the first derivative of cephalosporin to introduce the methoxyimino and aminothiazole groups at the 7 position of the cephem nucleus. Although it shows unexpectedly low oral absorption, its excellent activity against a wide range of gram-positive and gram-negative organisms, including Serratia, Enterobacter, Citrobacter, and anaerobes, guided research and development of the newer synthetic cephems, the so-called thirdgeneration cephalosporins.
Cefotaxime sodium salt acts as a beta-lactamase resistant antibiotic. It is used as an effective antibacterial against gram-negative bacteria, with the notable exception of pseudomonas and penicillin-resistant strains of streptococcus pneumoniae. It is used to treat infections of the bones, joints, skin, respiratory tract and blood stream.
Safety
| Symbol(GHS) |  GHS08 |
| Signal word | Danger |
| Hazard statements | H317-H334 |
| Precautionary statements | P261-P280-P284-P304+P340-P333+P313-P342+P311 |
| Hazard Codes | Xn,Xi |
| Risk Statements | 42/43 |
| Safety Statements | 22-36/37-60-45-37-24 |
| WGK Germany | 2 |
| RTECS | XI0250000 |
| HS Code | 29419000 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Resp. Sens. 1 Skin Sens. 1 |