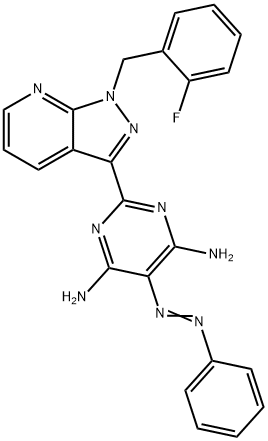
Riociguat , ≥99% , 625115-55-1
CAS NO.:625115-55-1
Empirical Formula: C20H19FN8O2
Molecular Weight: 422.42
MDL number: MFCD19443708
EINECS: 641-755-1
| Pack Size | Price | Stock | Quantity |
| 5MG | RMB415.20 | In Stock |
|
| 10MG | RMB711.20 | In Stock |
|
| 50MG | RMB727.20 | In Stock |
|
| 100mg | RMB1360.80 | In Stock |
|
| 500mg | RMB3252.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 247-251°C (dec.) |
| Boiling point: | 567.2±50.0 °C(Predicted) |
| Density | 1.51 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| pka | 1.51±0.50(Predicted) |
| form | Solid |
| color | Off-White to Orange |
| InChI | InChI=1S/C20H19FN8O2/c1-28(20(30)31-2)15-16(22)25-18(26-17(15)23)14-12-7-5-9-24-19(12)29(27-14)10-11-6-3-4-8-13(11)21/h3-9H,10H2,1-2H3,(H4,22,23,25,26) |
| InChIKey | WXXSNCNJFUAIDG-UHFFFAOYSA-N |
| SMILES | C(OC)(=O)N(C1=C(N)N=C(C2C3=CC=CN=C3N(CC3=CC=CC=C3F)N=2)N=C1N)C |
Description and Uses
Riociguat is a first-in-class oral medication that adds to the treatment options for PAH. In September 2013, Health Canada approved riociguat (also referred to as BAY 63-2521), for the treatment of patients with chronic thromboembolic pulmonary hypertension (CTEPH) after surgical treatment or inoperable CTEPH and for the treatment of adults with pulmonary arterial hypertension (PAH). Riociguat has a dual mode of action and works by (a) sensitizing sGC to the body's NO by stabilizing NO–sGC binding and (b) an NO-independent, direct stimulation of sGC via a different binding site. This process restores the NO–sGC–cGMP pathway and leads to increased generation of cGMP with subsequent vasodilation.
Headache, dizziness, dyspepsia/gastritis, nausea, diarrhea, hypotension, vomiting, anemia, gastroesophageal reflux, and constipation were the most common adverse events ( 3%) observed during riociguat clinical trials. Riociguat comes with a black box warning for embryo-fetal toxicity.
Riociguat is used in the treatment for pulmonary hypertension.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| WGK Germany | WGK 3 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Oral Aquatic Chronic 1 Repr. 2 |

![1-(2-fluoro-benzyl)-1h-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride](https://img.chemicalbook.com/CAS/GIF/256499-19-1.gif)
![2-(1-(2-Fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)pyrimidine-4,5,6-triamine](https://img.chemicalbook.com/CAS/20150408/GIF/428854-24-4.gif)