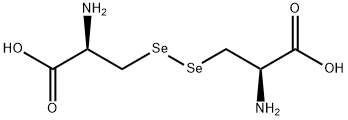
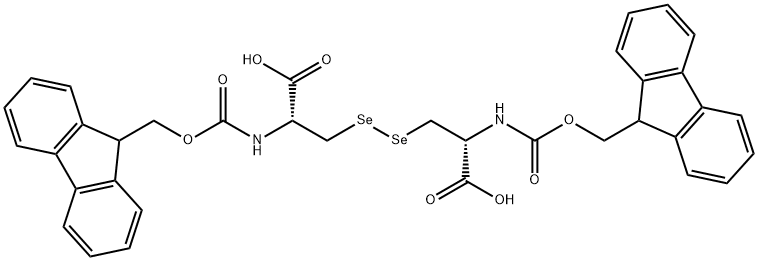
Seleno-<SC>L</SC>-cystine , 95% , 29621-88-3
Synonym(s):
(R,R)-3,3′-Diseleno-bis(2-aminopropionic acid);L -Selenocystine
CAS NO.:29621-88-3
Empirical Formula: C6H12N2O4Se2
Molecular Weight: 334.09
MDL number: MFCD00800971
EINECS: 608-382-6
| Pack Size | Price | Stock | Quantity |
| 50MG | RMB183.20 | In Stock |
|
| 250MG | RMB639.20 | In Stock |
|
| 1G | RMB1164.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 224.5-229.5 °C (lit.) |
| Boiling point: | 538.3±60.0 °C(Predicted) |
| storage temp. | 2-8°C |
| solubility | Aqueous Acid (Slightly), Aqueous Base (Slightly) |
| pka | 1.69±0.10(Predicted) |
| form | Powder |
| color | Yellow |
| optical activity | [α]20/D 28°, c = 1 in NaOH |
| BRN | 1969559 |
| Stability: | Hygroscopic |
| Major Application | peptide synthesis |
| InChI | 1S/C6H12N2O4Se2/c7-3(5(9)10)1-13-14-2-4(8)6(11)12/h3-4H,1-2,7-8H2,(H,9,10)(H,11,12)/t3-,4-/m0/s1 |
| InChIKey | JULROCUWKLNBSN-IMJSIDKUSA-N |
| SMILES | N[C@@H](C[Se][Se]C[C@H](N)C(O)=O)C(O)=O |
Description and Uses
L-Selenocystine is a diselenide-bridged amino acid that may be confused with selenocysteine (Sec), which is a rare amino acid featuring a single selenium atom. L-Selenocystine is a redox-active selenium compound that has both anti- and pro-oxidant actions. This compound can be reduced by low molecular thiols and disulfide reductases to Sec. It is reduced to Sec by mammalian thioredoxin reductase (apparent Km = 6.0 μM), and this property can be used to assay thioredoxin reductase activity. L-Selenocystine induces an unfolded protein response, ER stress, and large cytoplasmic vacuolization in HeLa cells and has cytostatic effects in a range of cancer cell types.
Seleno-L-cystine can be used for the synthesis of:
- Biologically active selenol compounds.
- Non-natural selenylated diamino acids.
Safety
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H301+H331-H373-H410 |
| Precautionary statements | P273-P301+P310+P330-P304+P340+P311-P314 |
| PPE | Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges |
| Hazard Codes | T,N |
| Risk Statements | 23/25-33-50/53 |
| Safety Statements | 20/21-28-45-60-61 |
| RIDADR | UN 3283 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | AY6032000 |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29319090 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Inhalation Acute Tox. 3 Oral Aquatic Acute 1 Aquatic Chronic 1 STOT RE 2 |