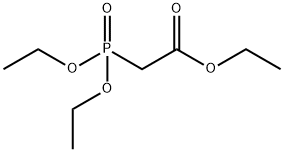
Triethyl phosphonoacetate , 98% , 867-13-0
Synonym(s):
(Diethoxyphosphinyl)acetic acid ethyl ester;Diethyl ethoxycarbonylmethylphosphonate;NSC 13898;NSC 16128;Triethyl carboxymethylphosphonate
CAS NO.:867-13-0
Empirical Formula: C8H17O5P
Molecular Weight: 224.19
MDL number: MFCD00009177
EINECS: 212-757-6
| Pack Size | Price | Stock | Quantity |
| 25G | RMB23.20 | In Stock |
|
| 100G | RMB33.60 | In Stock |
|
| 500G | RMB102.40 | In Stock |
|
| 2.5KG | RMB423.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | -24°C |
| Boiling point: | 142-145 °C9 mm Hg(lit.) |
| Density | 1.13 g/mL at 25 °C(lit.) |
| vapor pressure | 0.61Pa at 25℃ |
| refractive index | n |
| Flash point: | 165°C |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Liquid |
| color | Clear |
| Specific Gravity | 1.130 |
| Water Solubility | Slightly miscible with water. |
| BRN | 1343714 |
| InChI | 1S/C8H17O5P/c1-4-11-8(9)7-14(10,12-5-2)13-6-3/h4-7H2,1-3H3 |
| InChIKey | GGUBFICZYGKNTD-UHFFFAOYSA-N |
| SMILES | CCOC(=O)CP(=O)(OCC)OCC |
| LogP | 1.13 at 30℃ |
| CAS DataBase Reference | 867-13-0(CAS DataBase Reference) |
| EPA Substance Registry System | Acetic acid, (diethoxyphosphinyl)-, ethyl ester (867-13-0) |
Description and Uses
Triethyl phosphonoacetate is a reagent for organic synthesis used in the Horner-Wadsworth-Emmons reaction (HWE) or the Horner-Emmons modification. This compound can be added dropwise to sodium methoxide solution to prepare a phosphonate anion. It has an acidic proton that can easily be abstracted by a weak base. When used in an HWE reaction with a carbonyl, the resulting alkene formed is usually the E alkene and is generated with excellent regioselectivity. It can synthesize β-Keto Phosphonates via an acylation reaction of triethyl phosphonoacetate with carboxylic acid chlorides in the presence of magnesium chloride-triethylamine followed by decarbethoxylation. Acylation of triethyl phosphonoacetate using magnesium ethoxide affords acyl phosphonoacetates, which, on treatment with catalytic p-TsOH in water, are converted into 2-aryl-2-oxoalkylphosphonates[1-2].
Triethyl phosphonoacetate is used for Horner-Emmons modification.
Safety
| Symbol(GHS) |   GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H319-H411 |
| Precautionary statements | P264-P273-P280-P305+P351+P338-P337+P313-P391 |
| PPE | Eyeshields, Gloves, multi-purpose combination respirator cartridge (US) |
| Hazard Codes | N,Xi,Xn |
| Risk Statements | 51/53-36/37/38 |
| Safety Statements | 61-37/39-26-36 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 3 |
| RTECS | AG9800000 |
| Hazard Note | Harmful |
| TSCA | TSCA listed |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 29310095 |
| Storage Class | 10 - Combustible liquids |
| Hazard Classifications | Eye Irrit. 2 |

![2-(((3aR,4S,6R,6aS)-6-(7-aMino-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyriMidin-3-yl)-2,2-diMethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl)oxy)ethanol](https://img.chemicalbook.com/CAS/20150408/GIF/CB02668497.gif)