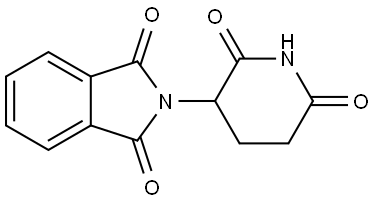
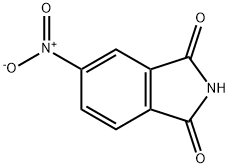
Thalidomide , ≥98% , 50-35-1
Synonym(s):
(±)-2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione;(±)-Thalidomide - CAS 50-35-1 - Calbiochem;(±)-2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione;(±)-Thalidomide
CAS NO.:50-35-1
Empirical Formula: C13H10N2O4
Molecular Weight: 258.23
MDL number: MFCD00153873
EINECS: 200-031-1
| Pack Size | Price | Stock | Quantity |
| 250mg | RMB84.80 | In Stock |
|
| 1G | RMB188.80 | In Stock |
|
| 5G | RMB414.40 | In Stock |
|
| 25g | RMB812.00 | In Stock |
|
| 100g | RMB2079.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 269-271°C |
| Boiling point: | 401.48°C (rough estimate) |
| Density | 1.2944 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 0.6 mg/mL |
| form | White solid |
| pka | 10.70±0.40(Predicted) |
| color | white |
| Water Solubility | <0.1 g/100 mL at 22 ºC |
| λmax | 300nm(lit.) |
| Merck | 14,9255 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChI | 1S/C13H10N2O4/c16-10-6-5-9(11(17)14-10)15-12(18)7-3-1-2-4-8(7)13(15)19/h1-4,9H,5-6H2,(H,14,16,17) |
| InChIKey | UEJJHQNACJXSKW-UHFFFAOYSA-N |
| SMILES | O=C1CCC(N2C(=O)c3ccccc3C2=O)C(=O)N1 |
| CAS DataBase Reference | 50-35-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Phthalimide, n-(2,6-dioxo-3-piperidyl)-(50-35-1) |
| EPA Substance Registry System | Thalidomide (50-35-1) |
Description and Uses
(±)-Thalidomide is an immunomodulatory compound with diverse biological activities, including anticancer, anti-inflammatory, and teratogenic properties. It prevents polymorphonuclear leukocyte (PMN) chemotaxis when used at concentrations of 1, 10, and 100 μg/ml. (±)-Thalidomide increases IL-2-induced proliferation and IFN-γ production in primary human T cells in vitro. It enhances natural killer (NK) cell-mediated cytotoxicity in MM.1S multiple myeloma cells. Thalidomide (4 mg/animal) reduces lung IL-6, TGF-β, VEGF, angiopoietin-1, angiopoietin-2, and collagen type Iα1 expression, inhibits pulmonary angiogenesis, and attenuates fibrosis in a mouse model of bleomycin-induced pulmonary fibrosis. It induces apoptosis in primary human embryonic fibroblasts (EC50 = 8.9 μM) and induces limb and eye defects in chicken embryos (EC50 = 50 μg/kg egg weight). Formulations containing thalidomide have been used in the treatment of multiple myeloma and erythema nodosum leprosum (ENL) in non-pregnant individuals.
antiinfective (topical)
Safety
| Symbol(GHS) |   GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H301-H312-H360D |
| Precautionary statements | P201-P202-P264-P280-P301+P310-P302+P352+P312 |
| PPE | Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges |
| Hazard Codes | T |
| Risk Statements | 46-61-21-25-62-22 |
| Safety Statements | 53-22-26-36/37/39-45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | TI4375000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29337900 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Oral Acute Tox. 4 Dermal Repr. 1A |
| Hazardous Substances Data | 50-35-1(Hazardous Substances Data) |
| Toxicity | LD50 oral in mouse: 2gm/kg |
| Limited Quantities | 5.0 L (1.3 gallons) (liquid) or 5.0 kg (11 lbs) (solid) |
| Excepted Quantities | Max Inner Pack (30g or 30ml) and Max Outer Pack (1Kg or 1L) |