A8352812
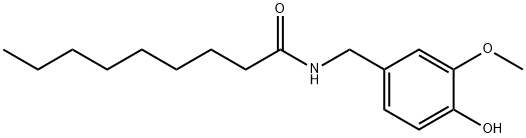
N-Vanillylnonanamide , 97% , 2444-46-4
Synonym(s):
Nonivamide;Pseudocapsaicin;Nonanoic acid vanillylamide;Capsaicin synthetic;N-(4-Hydroxy-3-methoxybenzyl)nonanamide
CAS NO.:2444-46-4
Empirical Formula: C17H27NO3
Molecular Weight: 293.4
MDL number: MFCD00017286
EINECS: 219-484-1
| Pack Size | Price | Stock | Quantity |
| 1G | RMB29.60 | In Stock |
|
| 5G | RMB60.00 | In Stock |
|
| 25G | RMB150.40 | In Stock |
|
| 100G | RMB568.80 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 54°C |
| Boiling point: | 200-210 °C(Press: 0.05 Torr) |
| Density | 1,1 g/cm3 |
| FEMA | 2787 | NONANOYL 4-HYDROXY-3-METHOXYBENZYLAMIDE |
| Flash point: | 190°C |
| storage temp. | Sealed in dry,2-8°C |
| solubility | methanol: 100 mg/mL, clear to slightly hazy |
| form | powder |
| pka | 9.76±0.20(Predicted) |
| color | white to off-white |
| Odor | bland odorless |
| Odor Type | bland |
| JECFA Number | 1599 |
| BRN | 2144300 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| Major Application | cleaning products cosmetics flavors and fragrances food and beverages personal care |
| Cosmetics Ingredients Functions | FRAGRANCE |
| InChI | 1S/C17H27NO3/c1-3-4-5-6-7-8-9-17(20)18-13-14-10-11-15(19)16(12-14)21-2/h10-12,19H,3-9,13H2,1-2H3,(H,18,20) |
| InChIKey | RGOVYLWUIBMPGK-UHFFFAOYSA-N |
| SMILES | CCCCCCCCC(=O)NCc1ccc(O)c(OC)c1 |
| LogP | 3.43 |
| CAS DataBase Reference | 2444-46-4(CAS DataBase Reference) |
| EPA Substance Registry System | Nonanamide, N-[(4-hydroxy-3-methoxyphenyl)methyl]- (2444-46-4) |
Description and Uses
Nonanoyl-4-hydroxy-3-methoxybenzylamide is odorless with a pungent, burning taste. Synthesized from nonanyl chloride and vanillylamine.
N-Vanillylnonanamide is a synthetic analogue of Capsaicin (175680) with similar bioactivity.
Safety
| Symbol(GHS) |    GHS05,GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H315-H317-H318-H334-H335 |
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 |
| target organs | Respiratory system |
| Hazard Codes | T,Xi |
| Risk Statements | 25-36/37/38-43 |
| Safety Statements | 26-45-37/39-36/37 |
| RIDADR | UN 3462 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | RA5998000 |
| F | 19 |
| TSCA | TSCA listed |
| HazardClass | 6.1 |
| PackingGroup | II |
| HS Code | 29242990 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Eye Dam. 1 Resp. Sens. 1 Skin Irrit. 2 Skin Sens. 1 STOT SE 3 |
| Toxicity | mouse,LD50,intraperitoneal,8mg/kg (8mg/kg),Journal of Medicinal Chemistry. Vol. 36, Pg. 2595, 1993. |