BD3383045
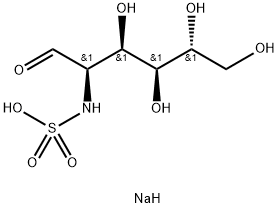
Sodium((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate , 95% , 38899-05-7
CAS NO.:38899-05-7
Empirical Formula: C6H12NNaO8S
Molecular Weight: 281.22
MDL number: MFCD00057972
EINECS: 609-596-2
| Pack Size | Price | Stock | Quantity |
| 25g | RMB26.40 | In Stock |
|
| 100g | RMB88.00 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in DMSO |
| InChI | InChI=1/C6H13NO8S.Na.H/c8-1-3(7-16(13,14)15)5(11)6(12)4(10)2-9;;/h1,3-7,9-12H,2H2,(H,13,14,15);;/t3-,4+,5+,6+;;/s3 |
| InChIKey | XWFHXUOTHFHRAS-IWNCBHIWNA-N |
| SMILES | [C@@H](C=O)(NS(O)(=O)=O)[C@@H](O)[C@H](O)[C@H](O)CO.[NaH] |&1:0,8,10,12,r| |
| CAS DataBase Reference | 38899-05-7(CAS DataBase Reference) |
Description and Uses
N-Sulfo-glucosamine sodium salt is a synthetic, high purity carbohydrate with a custom synthesis. It is an oligosaccharide that is also a sugar and a saccharide. The methylation of it can be achieved by glycosylation or click modification. Click modification is the addition of a carbon atom to the molecule through the reaction with an electrophile, such as N-hydroxysuccinimide ester. This modification can be used to introduce fluorine atoms into the molecules, which can improve their solubility and stability. The product has shown anti-inflammatory activities in animal models, which may be due to its ability to inhibit prostaglandin synthesis.
Safety
| WGK Germany | 3 |
| HS Code | 29329990 |