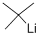LN7140529
1.3Msolutioninheptane,SpcSeal , 594-19-4
Synonym(s):
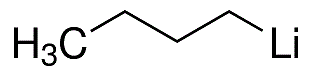
Lithium-2-methyl-2-propanide;t-BuLi
CAS NO.:594-19-4
Empirical Formula: C4H9Li
Molecular Weight: 64.06
MDL number: MFCD00008795
EINECS: 209-831-5
Update time: 2022-07-08
PRODUCT Properties
| Boiling point: | 36-40 °C |
| Density | 0.69 g/mL at 20 °C |
| vapor density | ~3 (vs air) |
| Flash point: | 20 °F |
| storage temp. | 2-8°C |
| form | liquid |
| color | slightly cloudy |
| Sensitive | Air & Moisture Sensitive |
| BRN | 3587204 |
| Exposure limits | ACGIH: TWA 1000 ppm OSHA: TWA 1000 ppm(2950 mg/m3) NIOSH: IDLH 1500 ppm; TWA 120 ppm(350 mg/m3); Ceiling 610 ppm(1800 mg/m3) |
| InChI | InChI=1S/C4H9.Li/c1-4(2)3;/h1-3H3; |
| InChIKey | BKDLGMUIXWPYGD-UHFFFAOYSA-N |
| SMILES | C(C)(C)(C)[Li] |
| CAS DataBase Reference | 594-19-4(CAS DataBase Reference) |
| EPA Substance Registry System | Lithium, (1,1-dimethylethyl)- (594-19-4) |
Description and Uses
tert-Butyllithium solution (tert-BuLi) is an organolithium compound, used as a strong base in organic chemistry. It facilitates lithium-halogen exchange reaction and also can be employed in the deprotonation of C-H compounds and amines. it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon molecules, including benzene. tert-Butyllithium is available commercially as hydrocarbon solutions; it is not usually prepared in the laboratory. Its synthesis was first reported by R. B. Woodward in 1941.
- There are no commercial uses of t-butyllithium, but it is used as a polymerization initiator and as a metalating agent in the laboratory.
- Alkylating and metalating agent. Reagent for the introduction of the tert-butyl group.
- tert-Butyllithium (t-BuLi) is the most reactive of the commercially available organolithium reagents. It is supplied as a standard solution in hydrocarbon solvents, usually in a bottle sealed with a septum.
Safety
| Symbol(GHS) |      GHS02,GHS05,GHS07,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H252-H318-H225-H250-H260-H304-H314-H336-H410-H411 |
| Precautionary statements | P301+P310a-P303+P361+P353-P305+P351+P338-P405-P422a-P501a-P210-P222-P223-P231+P232-P370+P378-P422 |
| target organs | Nervous system |
| Hazard Codes | F,C,N |
| Risk Statements | 11-15-17-34-51/53-65-66-67-50/53-38-14/15 |
| Safety Statements | 26-36/37/39-43-45-62-61-16-33-9 |
| RIDADR | UN 3394 4.2/PG 1 |
| WGK Germany | 1 |
| F | 3-10 |
| TSCA | TSCA listed |
| HazardClass | 4.3 |
| PackingGroup | I |
| HS Code | 29319090 |
| Storage Class | 4.2 - Pyrophoric and self-heating hazardous materials |
| Hazard Classifications | Aquatic Chronic 3 Asp. Tox. 1 Eye Dam. 1 Flam. Liq. 3 Pyr. Liq. 1 Repr. 2 Skin Corr. 1B STOT RE 1 Inhalation Water-react 1 |